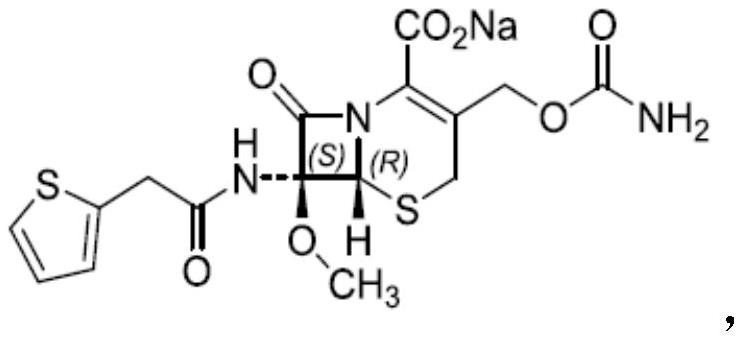
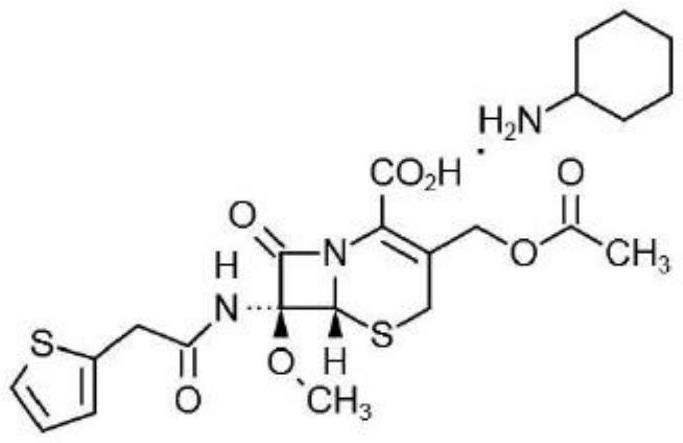
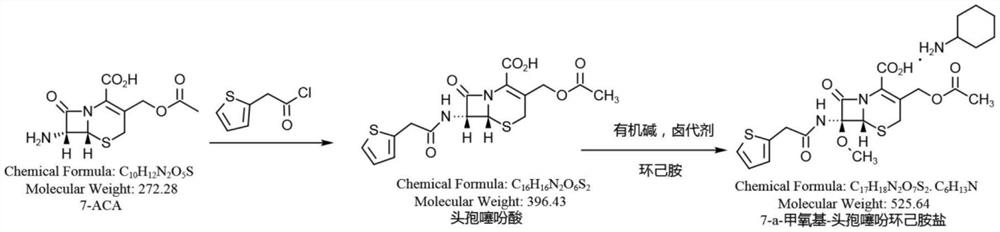
Synthesis method of cefoxitin sodium key intermediate
A technology of cefoxitin sodium and synthetic method, which is applied in the field of synthesis of key intermediates of cefoxitin sodium, and can solve the problems of low reaction yield and quality, unstable yield, unstable quality and yield of cefoxitin sodium, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Add 10.0g (36.76mmol) of 7-ACA to a 250ml three-necked flask, add a mixed solvent of 100ml water and 10mlEA (ethyl acetate) under stirring, heat up to 20°C, add 8.0g sodium bicarbonate (95.24mmol), and stir until Dissolve and clarify, cool down to 0°C, add 8.0g of 2-thiopheneacetyl chloride (49.84mmol) dropwise, react for 2h until the raw material remains ≤ 1.0%, raise the temperature to 25°C, add hydrochloric acid to adjust the pH to 1.5-2.5, filter to obtain cephalosporin Thiophenoic acid wet product. Add 100ml of dichloromethane to dissolve, separate layers to remove water, lower organic layer, cool to -60 ° C, add 30% sodium methoxide methanol solution 25.0g (138.89mmol), first add 2.8g tert-butyl hypochlorite (25.79mmol, tert-butyl hypochlorite: 7-ACA≈0.7:1), then detect the residual raw materials, calculate according to a certain method, add tert-butyl hypochlorite, after the raw materials are qualified, add acetic acid, add sodium chloride solution, add dilute Hy...
Embodiment 2
[0078]Add 10.0g (36.76mmol) of 7-ACA into a 250ml three-necked flask, add 120ml of water while stirring, raise the temperature to 25°C, add 7.0g of sodium bicarbonate (83.33mmol), stir until it dissolves and becomes clear, cool down to 15°C, add dropwise 6.0g 2-thiophene acetyl chloride (37.33mmol), react for 1h until the raw material residue ≤ 1.0%, raise the temperature to 30°C, add hydrochloric acid to adjust the pH to 1.5-2.0, filter to obtain the wet product of cephalothinic acid, and then add 200ml of dichloro Dissolve methane, separate layers to remove water, lower organic layer, cool to -90°C, add 30% sodium methoxide methanol solution 40.0g (222.22mmol), first add 3.5g tert-butyl hypochlorite (32.24mmol), and then detect raw materials Residue, calculated according to a certain method, add tert-butyl hypochlorite, add acetic acid, add sodium chloride solution, add dilute hydrochloric acid to control pH1.8-2.2, let stand to separate layers, concentrate and dry the lower ...
Embodiment 3
[0080] Add 10.0g (36.76mmol) of 7-ACA into a 250ml three-necked flask, add 100ml of dichloromethane while stirring, cool down to 0°C, add 9.0g of triethylamine (88.93mmol), stir until it dissolves and becomes clear, and add 7.0g of 2- Thiophene acetyl chloride (43.61mmol), reacted for 1.5h, until the raw material remained ≤0.5%, cooled to -80°C, added 30.0g (166.67mmol) of 30% sodium methoxide methanol solution, first added 4.0g tert-butyl hypochlorite ( 36.85mmol), then detect the residual raw materials, calculate according to a certain method, add tert-butyl hypochlorite, add acetic acid, add sodium chloride solution, add dilute hydrochloric acid to control PH1.8-2.2, let stand for stratification, add carbonic acid Sodium hydrogen, adjust pH 7.5, separate layers, add 100ml EA to the water layer, adjust pH 1.8 with hydrochloric acid, separate layers, dry the EA layer with anhydrous calcium chloride, add 5.2 g (52.44 mmol) of cyclohexylamine, crystallize, filter, Rinse with ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com