Current detection method of ion selective electrode
An ion-selective, current-detecting technology, applied in measurement devices, materials electrochemical variables, material analysis by electromagnetic means, etc. Long time, unfavorable rapid measurement and other problems, to achieve the effect of easy acquisition and configuration, highly sensitive measurement of signal amplification, and fast response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Characterization of potential of bare glassy carbon electrode as a function of calcium ion concentration when solid contact calcium ion selective electrode is used as reference electrode. Specifically include:
[0031] (1) Take two glassy carbon electrodes, respectively use 0.05μm Al 2 o 3 The powder is polished until the surface of the electrode is a bright mirror surface, and it is ultrasonically cleaned in ultrapure water, ethanol and ultrapure water in sequence, and it is used as a working electrode or an ion selective electrode matrix for later use;
[0032] (2) Take 60 μL of ordered mesoporous carbon solution (3 mg / mL) and drop-coat it on the glassy carbon electrode in step (1), and dry at room temperature to obtain ordered mesoporous carbon modified glassy carbon electrode;
[0033] (3) Weigh 4.14mg of N,N,N',N'-tetracyclohexyl-3-oxaglutaramide, 4.32mg of tetrakis(3,5-bis(trifluoromethyl)phenyl)sodium borate , 57.24 mg of polyvinyl chloride and 110 μL of 2-nit...
Embodiment 2
[0039] Current vs. voltage characterization of a potassium ferricyanide redox probe on a bare glassy carbon electrode. Specifically include:
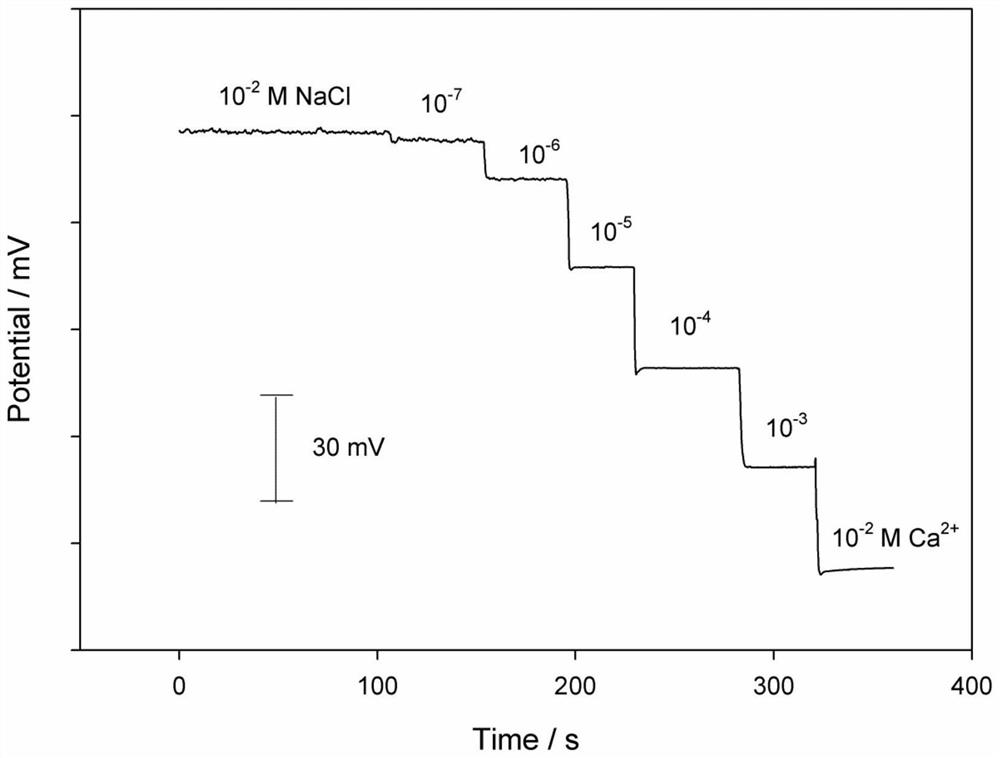
[0040] (1) Build according to the steps described in Example 1, the difference is that the step (5) device in Example 1 is adjusted, and the Ag / AgCl (3M KCl) electrode is placed on a 10 -2 In the beaker 2 of the M NaCl solution, the beaker 1 and the beaker 2 are connected by a salt bridge;
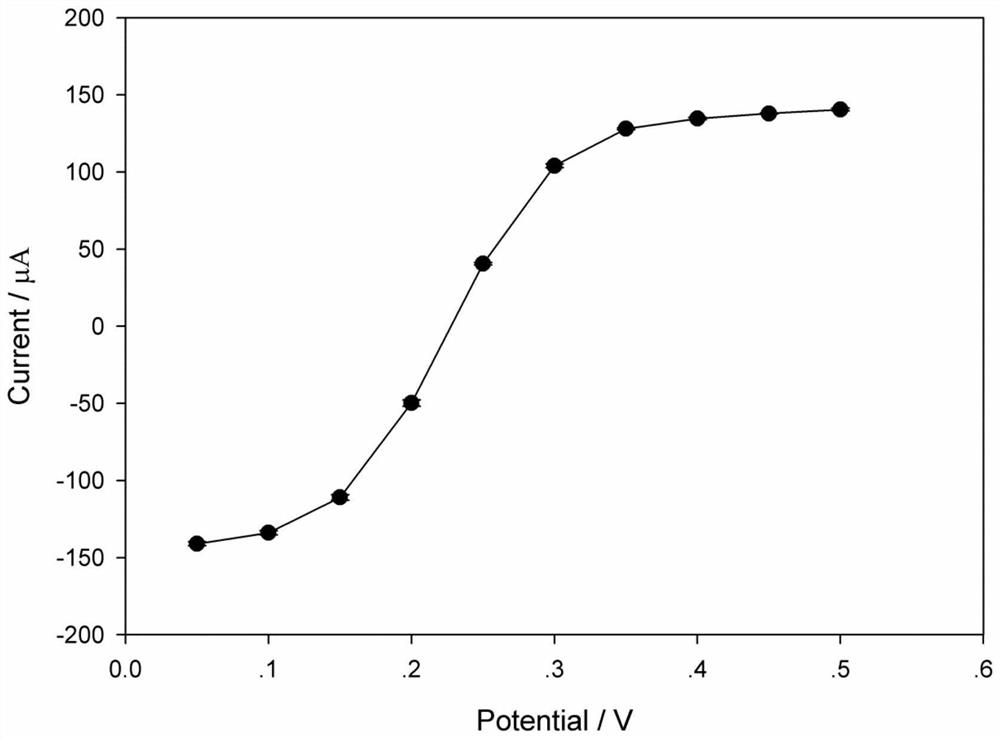
[0041] (2) Connect the three electrodes in step (1) to the CHI660C electrochemical workstation, select the multi-potential step technology, and set the voltage values in sequence: 0.05, 0.10, 0.15, 0.20, 0.25, 0.30, 0.35, 0.40, 0.45, 0.50V, the application time under each voltage is 20s, and the current of the potassium ferricyanide redox probe on the bare glassy carbon electrode changes with the voltage (see image 3 ).
[0042] Such as image 3 As shown, when the Ag / AgCl (3M KCl) commercial electrode is used as the reference electrode, the c...
Embodiment 3
[0044] Characterization of the current variation of the potassium ferricyanide redox probe on the bare glassy carbon electrode as a function of calcium ion concentration. Specifically include:
[0045] (1) Construct according to the steps described in Example 1, the difference is that the step (5) device in Example 1 is adjusted, a solution of 5 mM potassium ferricyanide and potassium ferrocyanide is placed in the beaker 1, and the three electrodes are connected Go to the CHI660C electrochemical workstation, select i-t technology, set the voltage between the bare glassy carbon electrode and the calcium ion electrode to -0.2V, gradually increase the calcium ion concentration in the solution, and obtain the potassium ferricyanide redox probe current with the calcium ion concentration. Change real-time response graph ( Figure 4 ), and obtain the corresponding calibration curve ( Figure 5 ).
[0046] Depend on Figure 4 and 5 As shown, with the increase of calcium ions, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com