Method for ratio-type electrochemical detection of nitrite ions
A nitrite ion and electrochemical technology, applied in the field of analytical chemistry, can solve the problems of strict detection environment conditions, complicated detection operation steps, expensive detection equipment, etc., and achieve low detection cost, mild conditions, and low-cost analysis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] OPD probe and NO 2 - Applied experiments to investigate the electrochemical behavior of
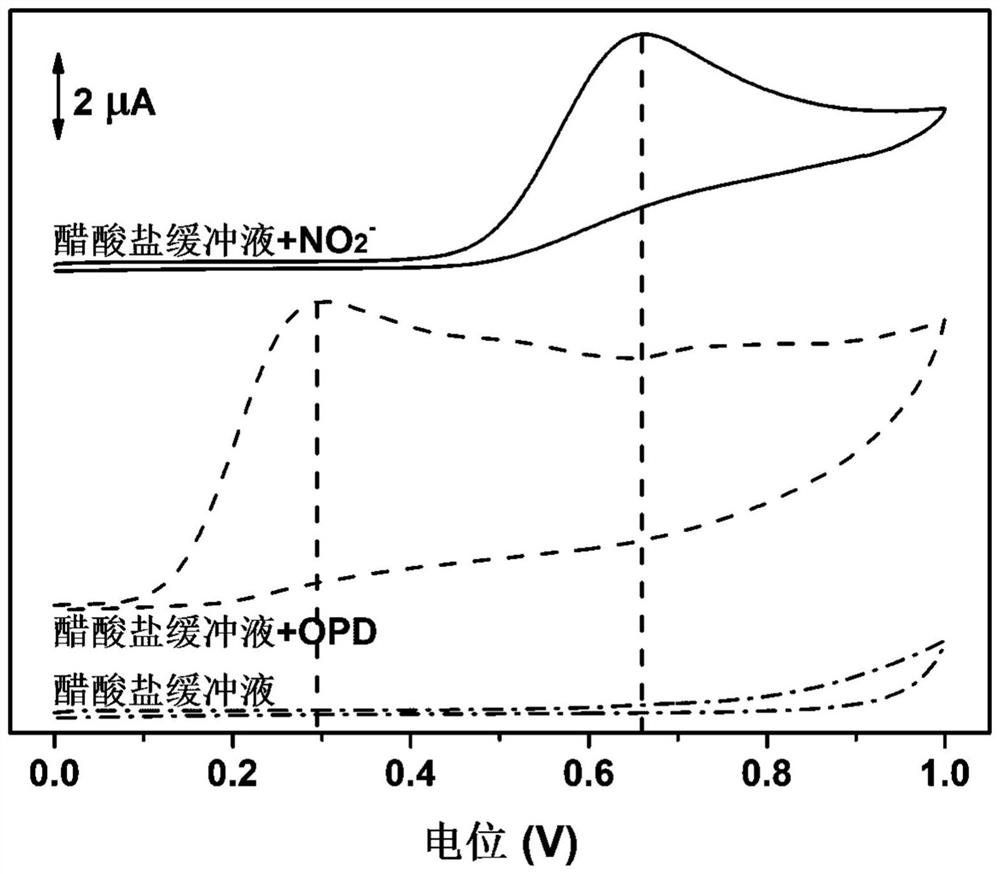
[0037] (1) Add 1000 μL of acetate buffer solution (0.1M) with pH=3 into a 1mL centrifuge tube, shake well, and measure the electrochemical signal by using the cyclic voltammetry (CV) of the electrochemical detection device (potential window: 0-1.0V; scan rate: 50mV / s);
[0038](2) Add 50 μL of 10 mM OPD solution to 950 μL of acetate buffer (0.1 M) at pH=3, shake well, and measure the electrochemical signal (potential window: 0-1.0V; scan rate: 50mV / s);
[0039] (3) Take 50μL of 5mM NO 2 - The solution was added to 950 μL of acetate buffer (0.1M) with pH=3, and after shaking well, the electrochemical signal was measured by cyclic voltammetry (CV) of the electrochemical detection device (potential window: 0-1.0V; scanning Rate: 50mV / s).
[0040] figure 1 Recorded for acetate buffer, acetate buffer + OPD, acetate buffer + NO 2 - The cyclic voltammogram. It can be seen from ...
Embodiment 2
[0042] NO 2 - For OPD probe +NO 2 - system impact
[0043] (1) Add 50 μL of 10 mM OPD solution and 900 μL of acetate buffer (0.1M) with pH=3 to a 1 mL centrifuge tube, and shake well;
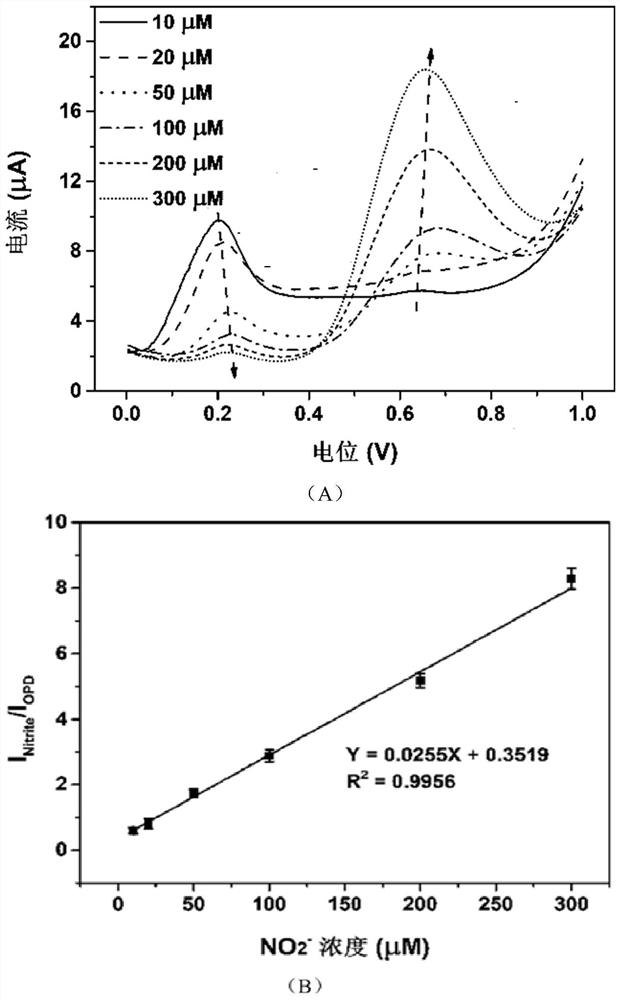
[0044] (2) Add 50 μL of different concentrations of NO to the above mixed solution 2 - The solution was shaken evenly, and after reacting for 1 min, the electrochemical signal was measured by linear sweep voltammetry (LSV) of the electrochemical detection device (potential window: 0-1.0V; scan rate: 50mV / s).
[0045] The result is as figure 2 as shown, figure 2 with NO 2 - With the increase of the concentration, the oxidation signal of OPD decreased gradually, and the NO 2 - The oxidation signal gradually increased. This is because the diazotization reaction between nitrite ions and OPD will occur in acidic medium, resulting in a decrease in the concentration of free OPD.
Embodiment 3
[0047] Different electrode pair OPD probe+NO 2 - Ratiometric electrochemical response of the system
[0048] (1) Add 50 μL of 10 mM OPD solution and 900 μL of acetate buffer (0.1M) with pH=3 to a 1 mL centrifuge tube, and shake well;
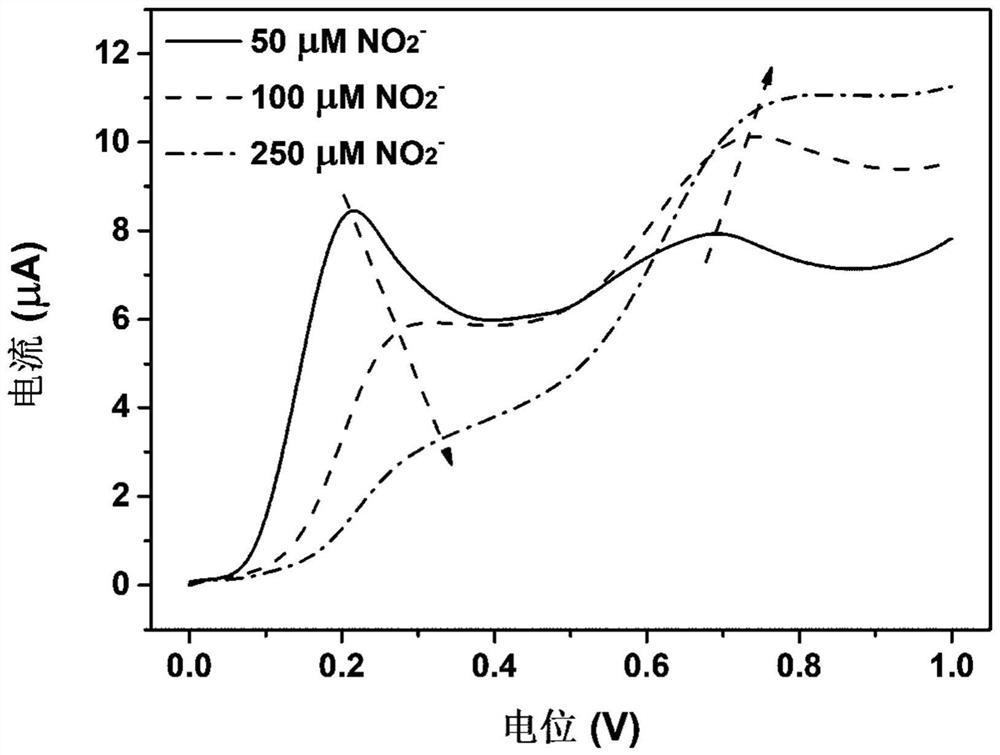
[0049] (2) Add 50 μL of 5 mM NO to the above mixed solution 2 - The solution was shaken evenly, and after reacting for 1 min, using different electrodes, the electrochemical signal was measured by differential pulse voltammetry (DPV) of the electrochemical detection device (initial potential: 0V; final potential: 1.0V; potential increase range: 4mV; amplitude : 50mV; pulse duration: 50ms; pulse period: 0.5s), record NO 2 - and the oxidation signal value of the OPD probe.
[0050] The result is as image 3 As shown, compared with the bare carbon electrode, the electrodes modified by Au, Pd and Pt are more effective for OPD probe + NO 2 - The ratiometric electrochemical response of the system has been improved to varying degrees, because ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com