Cytochalasin compound with immunosuppressive activity as well as preparation method and application of cytochalasin compound
A cytochalasin and compound technology, applied in the field of biomedicine, can solve problems such as killing of resting splenocytes, and achieve the effect of obvious immunosuppressive activity and low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1-compound
[0046] Phomopsis asparagi DHS-48 was inoculated into rice culture medium (100 g of rice, 3 g of coarse sea salt, 0.6 g of peptone, 100 mL of water, placed in a 500 mL Erlenmeyer flask), a total of 100 flasks were placed in 25 ° C for 28 days in the dark to obtain fermented product;
[0047] Pour the fermented product into an appropriate amount of ethyl acetate, ultrasonically extract for 30 minutes for the first time, and then soak and extract for 24 hours. The total fermentation product of the target bacterial strain was finally obtained, and the total fermentation product was dissolved and suspended with an appropriate amount of water, and the total fermentation product was dissolved and suspended with petroleum ether, dichloromethane, ethyl acetate, and n-butanol respectively. After extraction, petroleum ether extracts, dichloromethane extracts, ethyl acetate extracts and n-butanol extracts were obtained; the dichloromethan...
Embodiment 2
[0048] Example 2 Compound Structure Identification
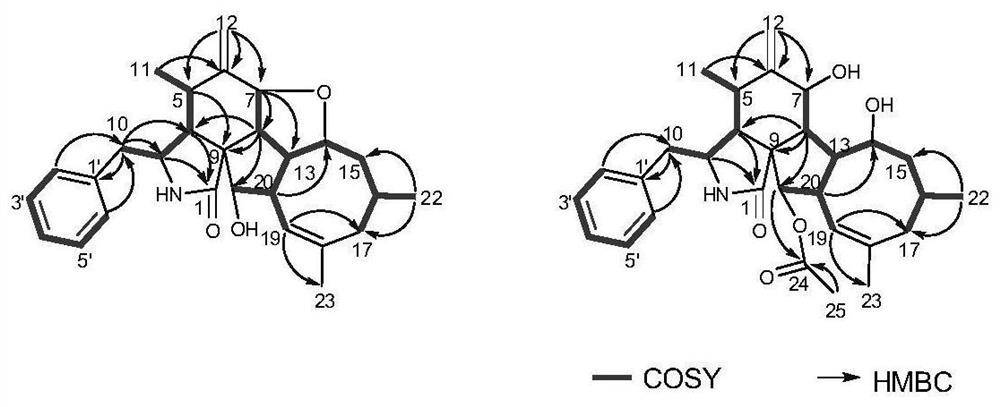
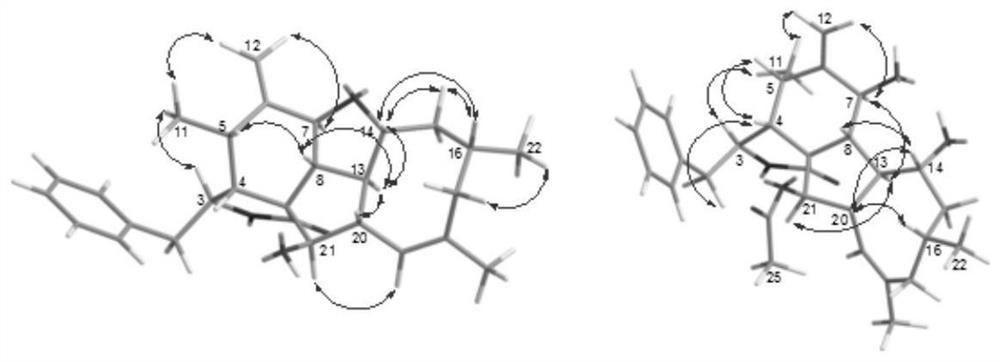
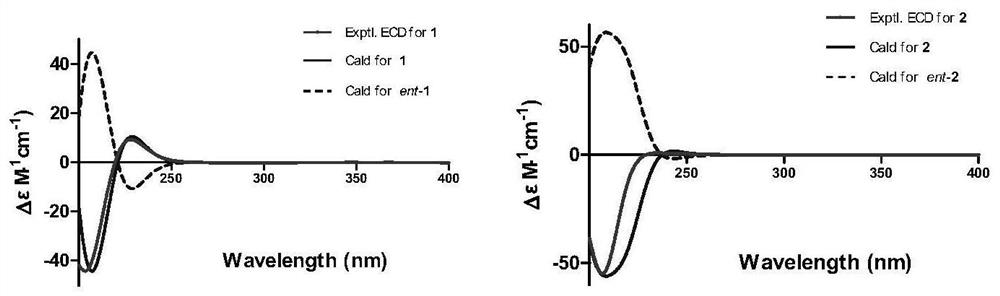
[0049] The plane structure and three-dimensional structure of the new compound were determined by analyzing its NMR, MS and electron circular dichroism (ECD) calculation data.
[0050] Compound 1, colorless amorphous powder, specific rotation [α] 25 D = (c 0.001, MeOH), UV absorbance UV(MeOH) λmax nm, cationic HR-ESI-MS gives [M+H] at m / z 434.2644 + peak, so the molecular formula of this compound is C 28 h 35 NO 3 , with an unsaturation of 12. The proton spectral data of compound 1 and the coupling constant of the attached proton indicate that at δ H(1.74,3H,s,H 3 There is a tertiary methyl group at -23), at δ H (0.73,3H,d,J=6.1Hz,H 3 -11; 0.95,3H,d,J=6.7Hz,H 3 There are two secondary methyl groups at -22), at δ H (5.13 and 4.96,2H, both s,H 2 There is an exocyclic methylene at -12), at δ H There are three Oxymethine, at δ H There is an alkenyl methine at (5.28,1H,br s,H-19), at δ H (7.22-7.30,5H) has a typic...
Embodiment 3
[0055] Calmodulin phosphatase inhibitory activity of embodiment 3 compound 2
[0056] Experimental program:
[0057] Dilute CNA into a suitable enzyme solution for later use, take several 5ml test tubes and put them on ice, add 10 μL of drug and 10 μL of enzyme solution in turn and incubate on ice for 5 minutes, add 180 μL of assay solution and react in a water bath at 30°C for 20 minutes, add 1800 μL of stop solution Terminate the reaction, measure the OD on the 720 type spectrophotometer 410 value.
[0058] Blank control: 10μL Enzyme Diluent + 10μL Buffer
[0059] Enzyme: 10μL Enzyme + 10μL Buffer
[0060] Control group: 10 μL enzyme diluent + 10 μL medicine
[0061] Enzyme + drug: 10μL enzyme + 10μL drug
[0062] Note: Buffer is the solution used to dissolve the drug
[0063] Calculate the relative inhibition rate of drugs on CNA according to the following formula:
[0064] Relative inhibition rate (%)=[1-(OD 药+酶 -OD 药对照 ) / OD 酶 ]×100%
[0065] See the experimenta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com