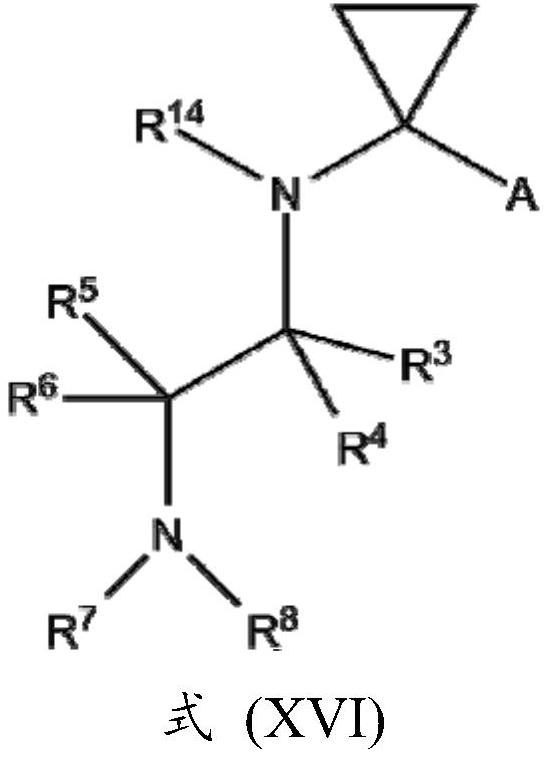
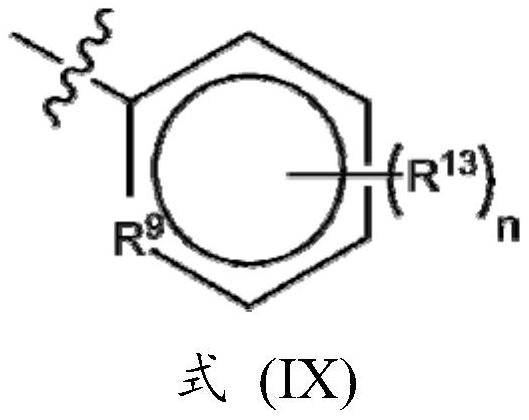
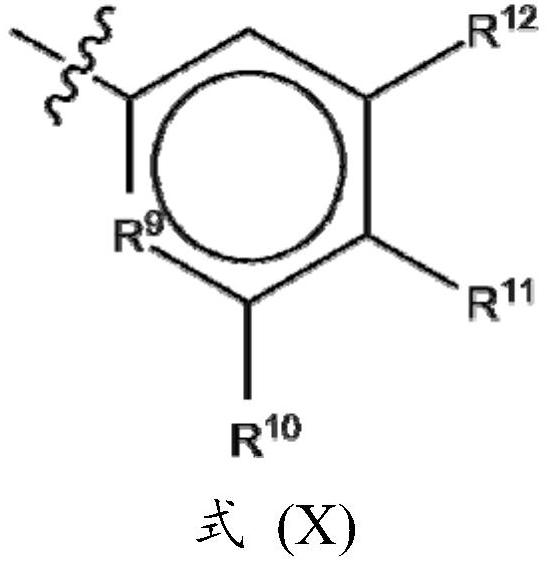
Novel potassium channel inhibitors
An alkyl and compound technology, applied in the field of new compounds, can solve problems such as poor solubility and achieve the effect of high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0304] Preparation of compounds
[0305] It may be (e.g., according to those in the working examples) were prepared according to any conventional chemical synthesis methods known to those skilled in the compounds according to the invention. Starting material for the method of the present application are known or can be readily prepared from commercially available chemicals by conventional methods known to the skilled person.
[0306] The final product of the reaction described herein can be separated by conventional techniques such as extraction, crystallization, distillation, chromatography, or the like.
[0307] The compounds of the invention may be present in a non-solvated form and a solvated form of a pharmaceutically acceptable solvent such as water, ethanol, or the like. Typically, for the purposes of the present invention, the solvate form is considered to be equivalent to the non-solventized form.
[0308] Pharmaceutical composition
[0309] The invention also relates to a...
Embodiment 1
[0627] Example [1] -N- (2- (dimethylamino) ethyl) -N- (1- (4-fluoro-3- (trifluoromethyl) phenyl) cyclopropyl) Methyl carbamate
[0628] step 1
[0629] The addition of 4-fluorine-3- (trifluoromethyl) benzoitrile (105.75 mmol) and isopropyl alcohol (IV) (116.33 mmol) in a solution of titanium (IV) (116.33 mmol) in dry ether in the ether in the ether. Based magnesium bromide 3M solution (222.09 mmol). The resulting yellow solution was stirred for 10 minutes and was slowly warmed to room temperature by 4h. Then, a trifluoride ethyl ether compound (211.51 mmol) was added and the reaction mixture was stirred at room temperature for 24 h. The reaction mixture was quenched with a 1.5 N HCl solution and extracted with ethyl acetate. The aqueous phase was alkated with a 10% sodium hydroxide solution and extracted with ethyl acetate. The organic layer was washed with brine and dried over anhydrous sodium sulfate to obtain a crude product: 10% TEA in acetate: petroleum ether was purified b...
Embodiment 2
[0634] Example [2] - (2-amino-2-methylpropyl) (1- (4-fluoro-3- (trifluoromethyl) phenyl) cyclopropyl) amino Sourmethyl
[0635] step 1
[0636] Diamparten oxidants were added to Solution of tert-butyl (20 g, 105.7 mmol) in dichloromethane (100 mL) at 0 ° C (1-hydroxy-2-methylpropylene)). Martin Periodinane (55.4 g, 126.8 mmol) and stirred at room temperature for 16 h. The reaction mixture was filtered and the filtrate was extracted with dichloromethane to wash with a saturated thiosulfate solution and 10% sodium bicarbonate solution. The organic layer was dried over sodium sulfate, filtered and dried under vacuum to give the crude product, and the ethyl acetate in petroleum ether was purified as a solvent to give a white solid (2-methyl-1). - oxo-2-yl) of tert-butyl ester [2.1] (19.5 g, 99%).
[0637] Step 2
[0638]To 1- (4-fluoro-3- (trifluoromethyl) phenyl) cyclopropylene-1-amine [1.1] (0.22 mmol) in IPA (5 mL) in IPA (5 mL) at 0 ° C Titcharide (2-methyl-1-oxpropan-2-yl) carb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com