Application of amino acid modified amino tetraphenylporphyrin compound in prevention and treatment of fibrosis
An aminotetraphenylporphyrin, amino acid technology, applied in the field of medicine, can solve the problems of poor prognosis of patients, lack of treatment methods, and high mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 Amino acid modified amino tetraphenyl porphyrin compound (LD 4 )Synthesis
[0048] Boc-Lys(Boc)-OH (467.67mg, 1.35mmol) was placed in the reaction flask, N 2 Add dry THF 20ml under protection, and stir with a magnet. Cool to -17°C, add triethylamine (197.60 μl, 1.42 mmol) and ethyl chloroformate (131.10 μl, 1.38 mmol) to react for 1 h, a white precipitate is formed, filter and discard the precipitate. Tetraaminoporphyrin (202.40mg, 0.30mmol) was dissolved in 15ml THF, the above filtrate was added, and the reaction was stirred at room temperature for 14h. TLC (dichloromethane:methanol:ammonia=60:1:0.6) monitored the progress of the reaction. After the reaction was complete, the reaction solution was poured into ice water to precipitate a precipitate, which was filtered and washed three times with water to obtain a purple solid. Finally, separation by column chromatography (eluent: dichloromethane: methanol: ammonia water = 30:1:0.4) yielded 596.13 mg of ...
Embodiment 2
[0050] Embodiment 2 Amino acid modified aminotetraphenylporphyrin compound (LD 4 ) to prevent and treat intestinal fibrosis
[0051] Amino acid-modified aminotetraphenylporphyrin compound LD prepared in Example 1 of the present invention 4 The in vivo experimental process of treating dextran sodium sulfate (DSS)-induced intestinal fibrosis in mice comprises the following steps:
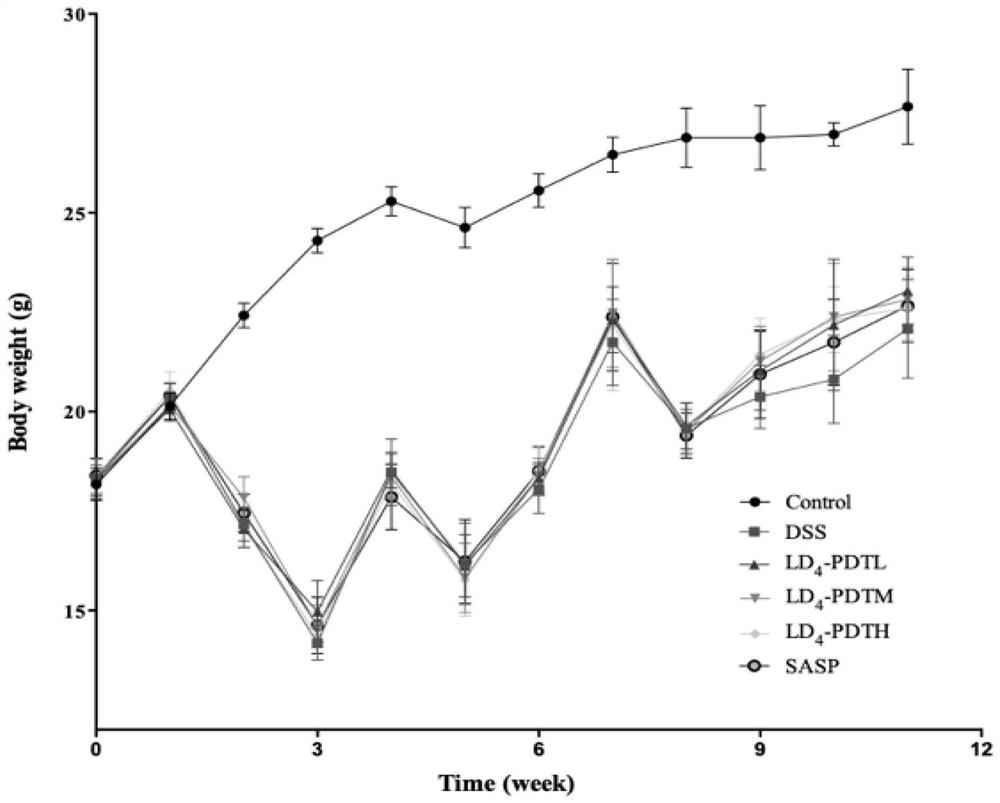
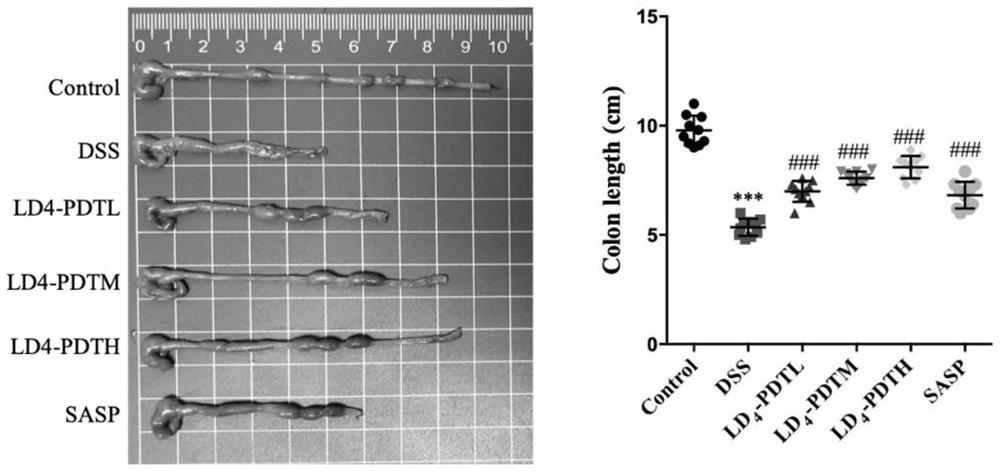
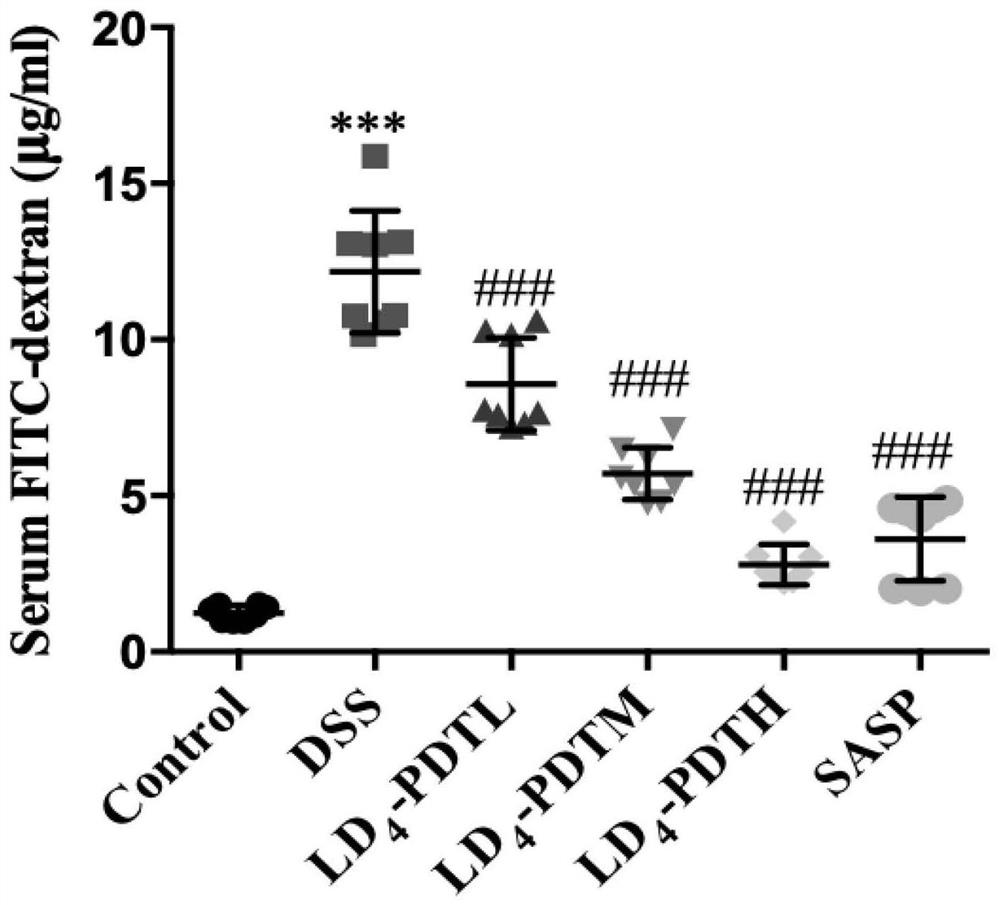
[0052] The normal group of C57BL / 6J mice drank purified water, and the rest of the groups drank 1.5% DSS water for one week, purified water for two weeks, followed by 2% DSS water for one week for two weeks, and then circulated 2% DSS water for one week to establish Intestinal fibrosis model. The mice were randomly divided into 6 groups, 10 in each group, and the treatment method was as follows: 1.Control group (normal saline); 2.DSS group (DSS drinking water); 3.LD 4 -PDTL group (DSS drinking water and low-dose LD 4 60μg / kg enema); 4.LD 4 -PDTM group (DSS drinking water and medium dose LD 4 1...
Embodiment 3
[0061] Embodiment 3 Amino acid modified aminotetraphenylporphyrin compound (LD 4 ) to prevent and treat intestinal fibrosis
[0062] Amino acid-modified aminotetraphenylporphyrin compound LD prepared in Example 1 of the present invention 4 The in vivo experimental process of treating trinitrobenzenesulfonic acid (TNBS)-induced intestinal fibrosis in rats comprises the following steps:
[0063] Rats were fasted for 24 h before model induction and anesthetized with 10% chloral hydrate. Intestinal fibrosis model was established by injecting 150 mg / kg TNBS into the colon of rats with a 3 mm diameter polyethylene rubber catheter (inserted 8 cm proximal to the anorectum). The rats were randomly divided into 6 groups, 6 in each group, and the treatment method was as follows: 1.Control group (normal saline); 2.TNBS group (TNBS enema); 3.LD 4 -PDTL group (TNBS and low-dose LD 4 , 60μg / kg, both enema); 4.LD 4 -PDTM group (TNBS and medium dose LD 4 , 120μg / kg, both enema); 5.LD 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com