Method for separating and determining posaconazole Z2 and impurities thereof
A technology for posaconazole and impurities, which is applied in the field of analytical chemistry, can solve problems such as insufficient precision, undisclosed methods for qualitative and quantitative analysis of posaconazole by liquid chromatography-mass spectrometry, and achieve the effect of improving the recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1 blank diluent verification
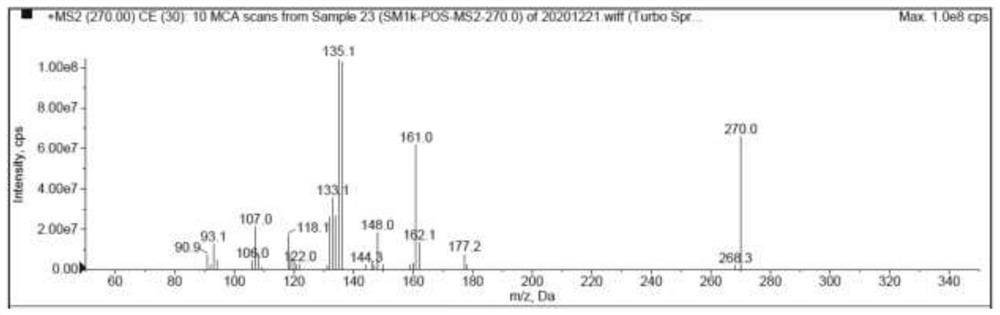
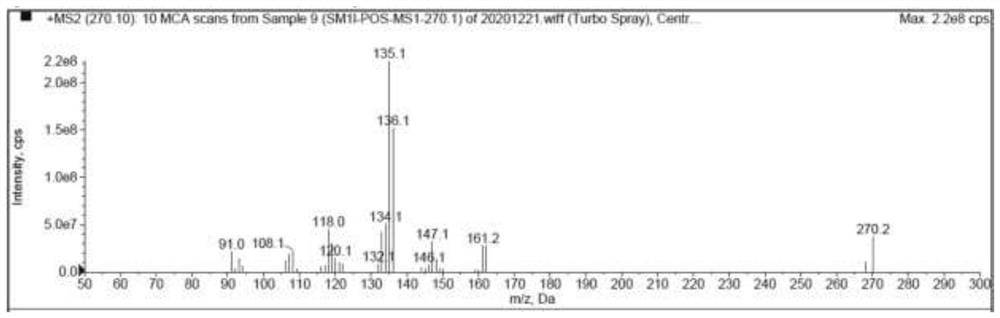
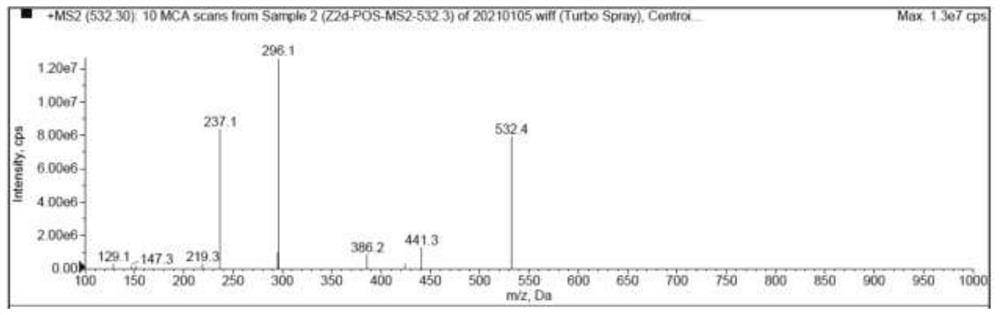
[0095] Use blank solvent to mix with impurity SM respectively 1l , SM 1k ,Z 2d and Z 2h Enter the liquid chromatography for analysis, and the analysis results are as follows: Figure 5-Figure 8 As shown, the blank solvent is in SM 1l , SM 1k ,Z 2d and Z 2h There is no chromatographic peak at the peak retention time, and there is no interference to the detection of impurities.
Embodiment 2
[0096] Example 2 Repeatability Verification of Reference Substance Solution
[0097] Prepare SM 1l , SM 1k ,Z 2d and Z 2h The reference substance solution, and accurately measure 20 μ l into the high-performance liquid chromatography-mass spectrometer, each impurity was injected 6 times, and the chromatographic data are shown in Table 3 below, wherein the chromatogram of the sixth injection of each impurity Such as Figure 9-Figure 12 Shown, as can be seen from Table 3, the reference substance solution injection repeatability is good.
[0098] Table 3 Impurity reference substance solution chromatogram situation
[0099]
[0100]
Embodiment 3
[0101] Example 3 Quantitative Limit Verification
[0102] For impurities SM 1k , SM 1l ,Z 2d and Z 2h Carry out quantitative limit test, test result is as shown in table 4 below, and its chromatogram is as follows Figure 13-15 As can be seen from the table, SM 1k , SM 1l ,Z 2d and Z 2h The quantitation limit concentrations are about 0.710ng / ml, 0.698ng / ml, 0.747ng / ml and 0.727ng / ml respectively, the quantitation limits are about 0.710ppm, 0.698ppm, 0.747ppm and 0.727ppm respectively, and the RSDs of the peak areas are all less than 15%, and the signal-to-noise ratio is greater than 10.
[0103] The limit of quantitation detection situation of table 4 impurity
[0104]
[0105]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Snr | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com