Preparation method and application of microenvironment response type polymer prodrug co-delivery gel system
A polymer, responsive technology, applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of low selectivity, limited clinical application, poor water solubility, etc. Clear, high drug utilization, high drug loading effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
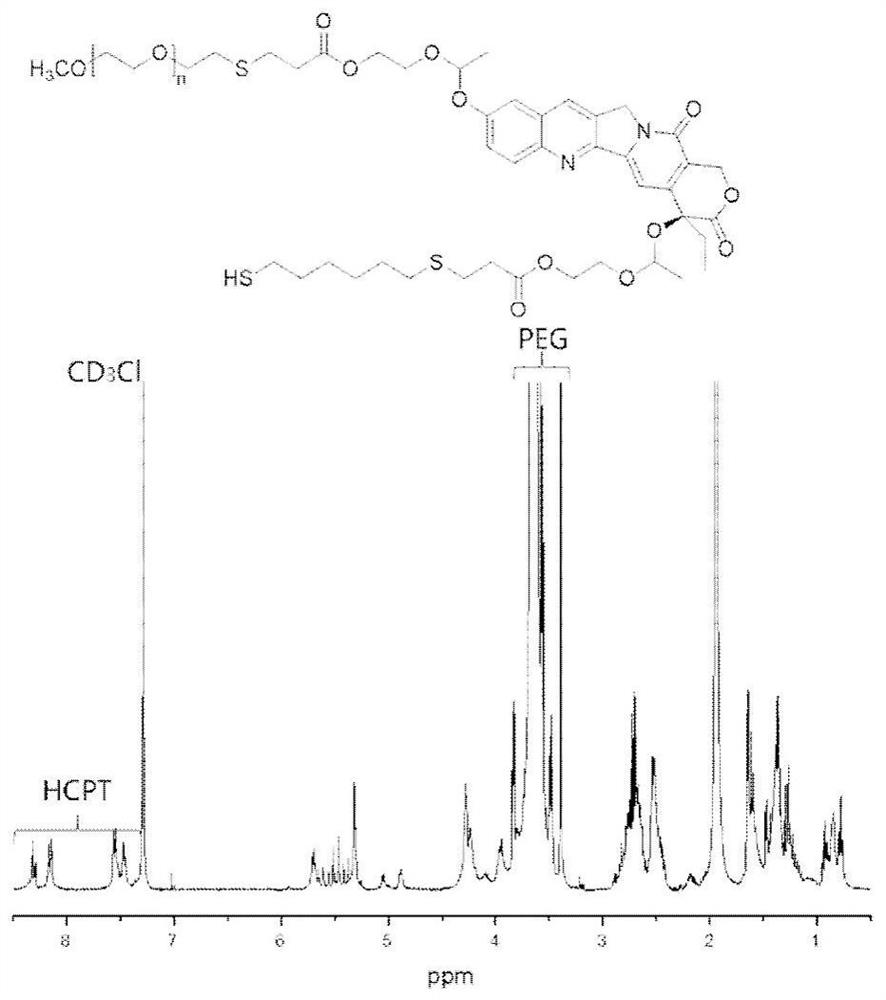
[0038] Example 1 Preparation of thiolated acid-responsive polymer prodrug
[0039]
[0040] 1,6-Hexanedithiol (22 μL, 0.143 mmol) was dissolved in N,N-dimethylformamide (DMF), and hydroxycamptothecin-vinyl ether acrylate-polyethylene oxide was added dropwise under nitrogen protection. Ethylene glycol complex (100 mg, 0.0357 mmol) was dissolved in DMF, and triethylamine (TEA) was added, and the reaction was stirred overnight at room temperature. After the reaction, the reaction liquid was precipitated with glacial ether, and the yield was 93.24%. Proton NMR spectrum such as figure 1 shown.
Embodiment 2
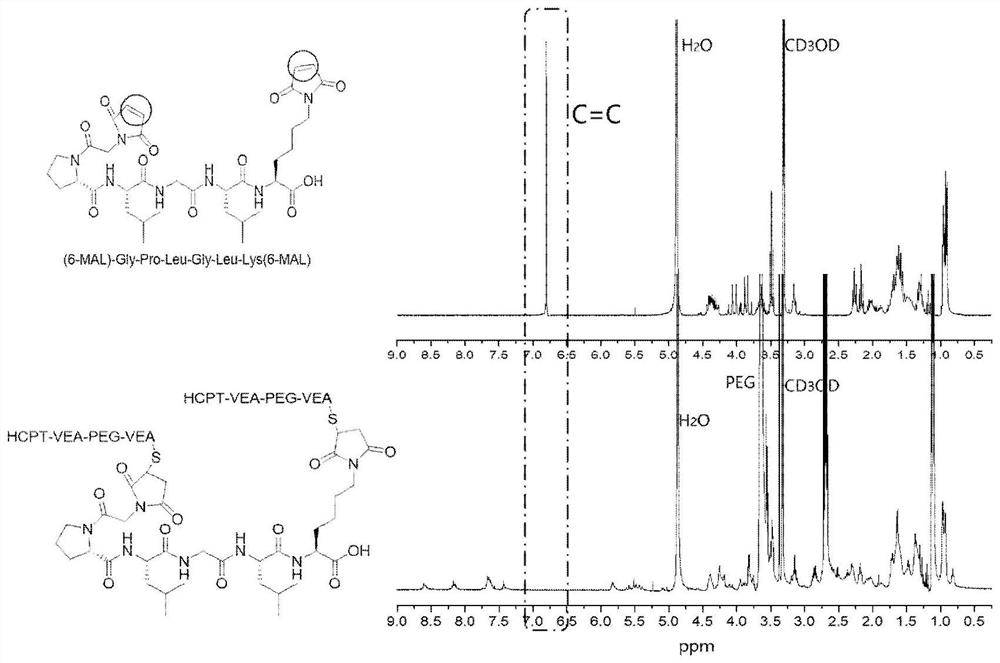
[0041] Example 2 Assembly and crosslinking of microenvironment-responsive polymer prodrugs
[0042] The polymer prodrug (1.0 mg, 0.357 μmol) was dissolved in absolute ethanol, and the solution was slowly added dropwise to high-purity water under ultrasonic conditions. Drug micelles. Polymer prodrug (1.0mg, 0.357μmol) was dissolved in absolute ethanol, mixed with ethanol solution (0.208mg, 0.214μmol) of MMP-9 sensitive polypeptide under nitrogen protection, then added triethylamine, at room temperature Stirring and reacting for 4 hours, the reaction solution was slowly added dropwise into high-purity water under ultrasonic conditions, and the obtained solution was continued to be sonicated, and then dialyzed in high-purity water to obtain a cross-linked tumor microenvironment-responsive polymer prodrug gel.
[0043] figure 2 It is the proton nuclear magnetic spectrum of the MMP-9 sensitive polypeptide (top) and its cross-linking reaction with HCPT-VEA-PEG-SH (bottom). The r...
Embodiment 3
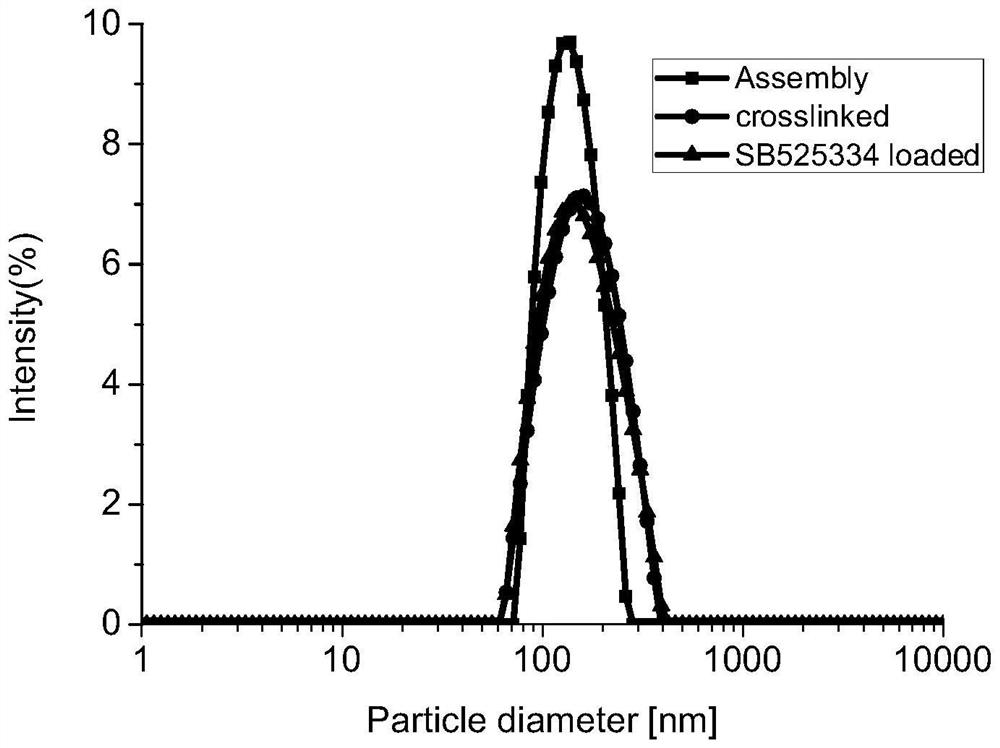
[0044] Example 3 Encapsulation of TGF-β inhibitors
[0045] Add 2 μl of TGF-β inhibitor ethanol solution (5 mg / mL) to 2 mL of microenvironment-responsive polymer prodrug gel aqueous solution (0.5 mg / mL), alternately perform vortex mixing and ultrasonication three times each to complete drug loading . image 3 It is the particle size diagram of the polymer prodrug gel before and after cross-linking and after cross-linking and loading TGF-β inhibitor SB525334. The average particle size of the material before crosslinking was 142nm, and the particle size distribution was 0.18; the average particle size after crosslinking was 168nm, and the particle size distribution was 0.23; the average particle size after loading TGF-β inhibitor did not change much, being 165nm, the particle size distribution is 0.24.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com