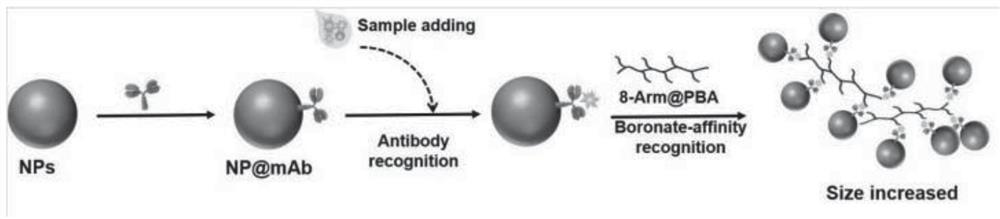
Glycoprotein dynamic light scattering immunization method based on phenylboronic acid cross-linking agent
A dynamic light scattering and glycoprotein technology, applied in the field of immunoassay, can solve the problems of false positive signal, low sensitivity, unstable signal, etc., and achieve the effect of simple operation steps, high sensitivity detection, and reduced demand.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Application of using magnetic nano-microspheres to detect the content of alpha-fetoprotein
[0041] 1 Preparation of carboxylated Fe3O4 magnetic microspheres
[0042] 1) Preparation of oleated Fe3O4 magnetic beads
[0043] a. Synthesis of Fe304 magnetic beads, add 300mL ultrapure water into a 500mL three-necked flask, pass through N2 to remove oxygen in the water, and preheat at 50°C for 20min, add 3.2gFeCl2·H20, 5.2gFeCl3 Magnetic stirring and mixing, then add 25mL Ammonia water, the yellow solution quickly turns black, react at a constant temperature of 50°C for 30 minutes, separate the synthesized magnetic beads by magnetic suction, wash 3-5 times with ultrapure water until the pH of the solution reaches neutral after magnetic absorption, and then redissolve in 300mL ultrapure water middle.
[0044] b. Oil acidification modification of Fe304 magnetic beads, add the magnetic beads in the previous step into a three-necked flask, pass through N2, add 2.4mL o...
Embodiment 2
[0059] Example 2 Glycoprotein-alpha-fetoprotein as the object to be detected
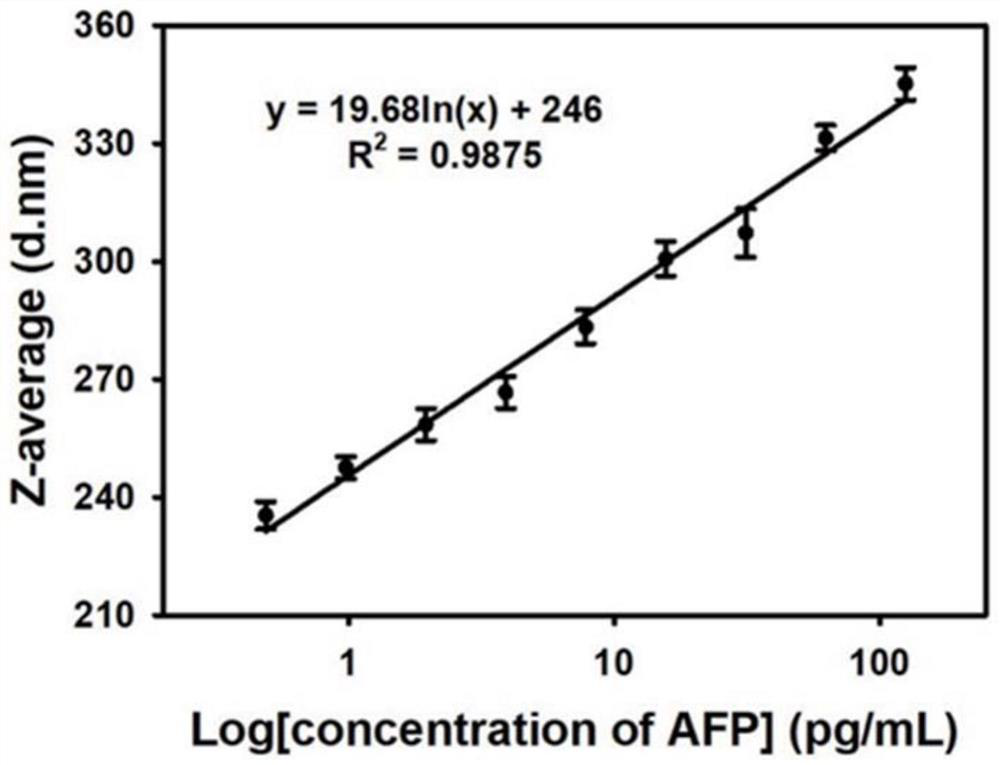
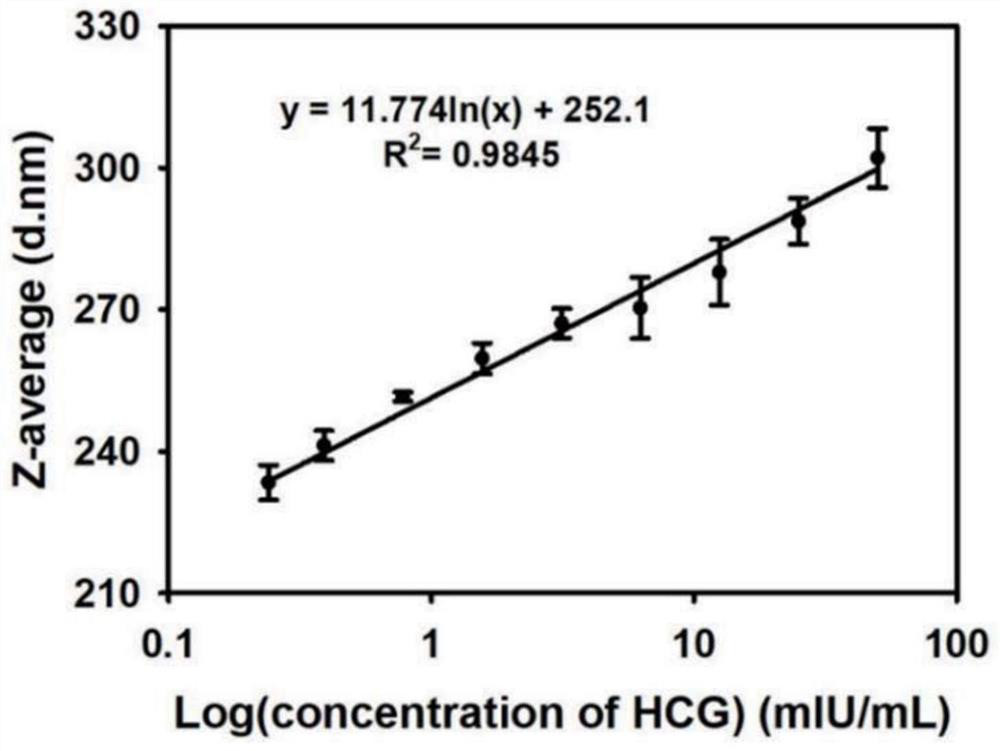
[0060] 6 μL of alpha-fetoprotein detection antibody-labeled Fe304 microsphere probe was reacted with 200 μL of different concentrations of alpha-fetoprotein samples for 5 minutes, magnetically sucked for 5 minutes, the supernatant was discarded and ultrasonically washed 2-3 times with PB7.5 (0.01mol / L), and then added 800μL of 0.2mg / mL 8arm PEG@3-APBA was reconstituted and sonicated, incubated at 25°C for 5min, taken out at 25°C and measured with a Malvern Nanoparticle Size Analyzer to measure the average hydration kinetic diameter of the solution, and calculated the average offspring Enter the standard curve to obtain the concentration of alpha-fetoprotein in the sample to be tested. The specific experimental results are as follows: the linear standard curve is y=19.68ln(x)+246, R=0.9875, see the attached figure 2 , the lowest detection limit of the method is defined as the average hydration parti...
Embodiment 3
[0061] Example 3 Application of gold magnetic microspheres to detect alpha-fetoprotein content
[0062] 1 Preparation of gold magnetic probe labeled with detection antibody
[0063] Take 10 μL of gold magnetic nanoparticles (10 mg / mL) and add them to 200 μL of pH8.0 PB, add 4 μg of the detection antibody corresponding to alpha-fetoprotein, stir and react at room temperature for 30 min; continue to stir the reaction, add 0.2 μg of 1-ethyl-(3-di After methylaminopropyl)-3-ethylcarbodiimide hydrochloride, stir at room temperature for 30 minutes, add twice, after the completion of the reaction, magnetic suction for 5 minutes, discard the supernatant, and then add acid with a mass volume fraction of 2% for hydrolysis casein, and added 0.2 μg 1-ethyl-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, stirred at room temperature for 1 h, and discarded the supernatant after magnetic absorption for 5 min, then added The 6-amino-3-pyridine phenylboronic acid with a mass volume ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com