Walnut meal acetylcholine esterase inhibiting peptide as well as preparation method and application thereof
A technology for acetylcholinesterase and inhibitory peptides, which is applied in the field of acetylcholinesterase inhibitory peptides and its preparation, can solve the problems of wasting walnut protein resources and hindering the development of walnut industry, and achieve low production costs, obvious enzyme inhibition, and high economic benefits Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the extraction method of walnut meal polypeptide
[0042] (1) Degreasing walnut meal:
[0043] The walnut pulp was crushed through a 40-mesh sieve, extracted with petroleum ether at a solid-liquid ratio (w / v) of 1:5 for 2 hours, filtered, and the residue was collected, extracted continuously for 3 times, and the residue was placed in a fume hood to evaporate the organic solvent. The degreased walnut meal powder was obtained and stored at 4°C for future use.
[0044] (2) Alkali-soluble acid precipitation to extract walnut protein
[0045] Accurately weigh 10g of degreased walnut meal powder into a 400mL beaker, add 200mL of distilled water, adjust the pH to 10.0, stir on a magnetic stirrer for 5min, then ultrasonicate for 20min, take it out immediately after the ultrasonication is over, and let it stand for 1h, 6000r / min Centrifuge for 20 min, and take the supernatant for later use. Use 1mol / L HCl to adjust the pH to 4.5, let it stand for 1h, and centrif...
Embodiment 2
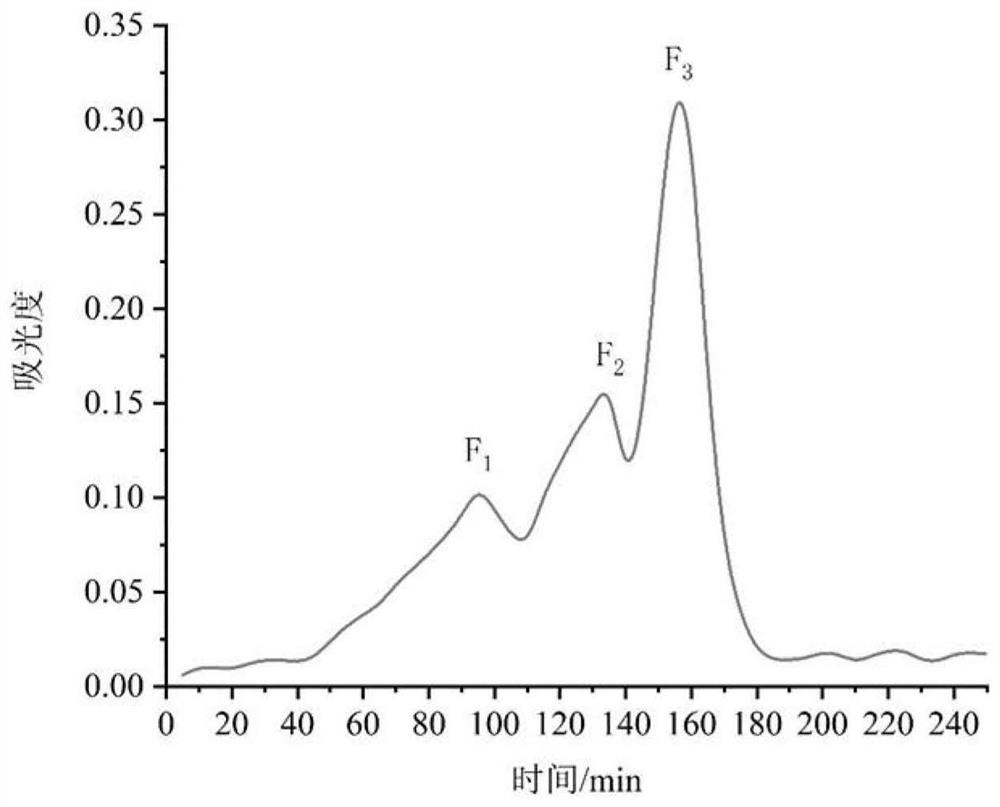
[0055] The inhibitory ability of walnut meal polypeptides with different molecular weights to acetylcholinesterase after the ultrafiltration of embodiment 2
[0056] The enzymolysis solution of walnut meal obtained by combined enzymolysis with alkaline protease and pepsin contains free amino acids, polypeptides of different lengths, and proteins with a small degree of hydrolysis, etc. purification.
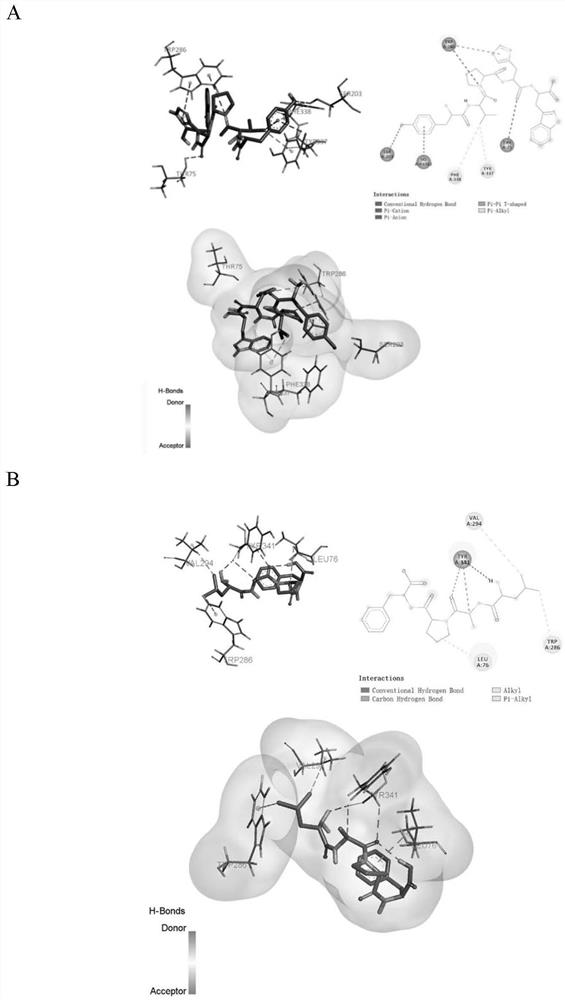
[0057] In this example, walnut meal polypeptides were purified by means of ultrafiltration, using acetylcholinesterase inhibitory ability as an indicator, and the polypeptide sequences of the purified components were identified by LC-MS / MS. Through PyRx virtual screening combined with Autodock Vina molecular docking and literature research, the peptides that may have strong acetylcholinesterase inhibitory ability were screened out.
[0058] (1) ultrafiltration
[0059] The enzymatic hydrolysis product of walnut meal prepared in Example 1 was subjected to ultrafiltration to purif...
Embodiment 3
[0084] Example 3 Verification of polypeptide activity
[0085] (1) Artificial synthesis of peptides
[0086] The synthesis of peptides adopts the Fmoc solid-phase synthesis method, and peptides are synthesized according to the amino acid sequence, and powdered peptides are obtained after cleavage, precipitation, and purification. The synthesis process was entrusted to Nanjing Yuanpeptide Biotechnology Co., Ltd. The purity of peptides YVPHW, LAPF and FYRR were all greater than 95%.
[0087] (2) Activity verification of artificially synthesized peptides
[0088] According to the method described in Example 1, the inhibitory rate of different concentrations of peptides to acetylcholinesterase was measured,
[0089] According to the fitting calculation of the experimental results, the half-inhibitory concentration (IC) of the peptide to acetylcholinesterase was calculated. 50 ). IC of peptide YVPHW 50 The value is 139.10±1.34 μg / mL, the IC of LAPF 50 The value is 318.80±1.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com