Method for synthesizing indene compound
A technology of compounds and acids, which is applied in the field of synthesizing indenes, can solve the problems of harmful by-products, danger-prone, harsh reaction conditions, etc., and achieve the effects of high conversion rate, wide applicability, and mild reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
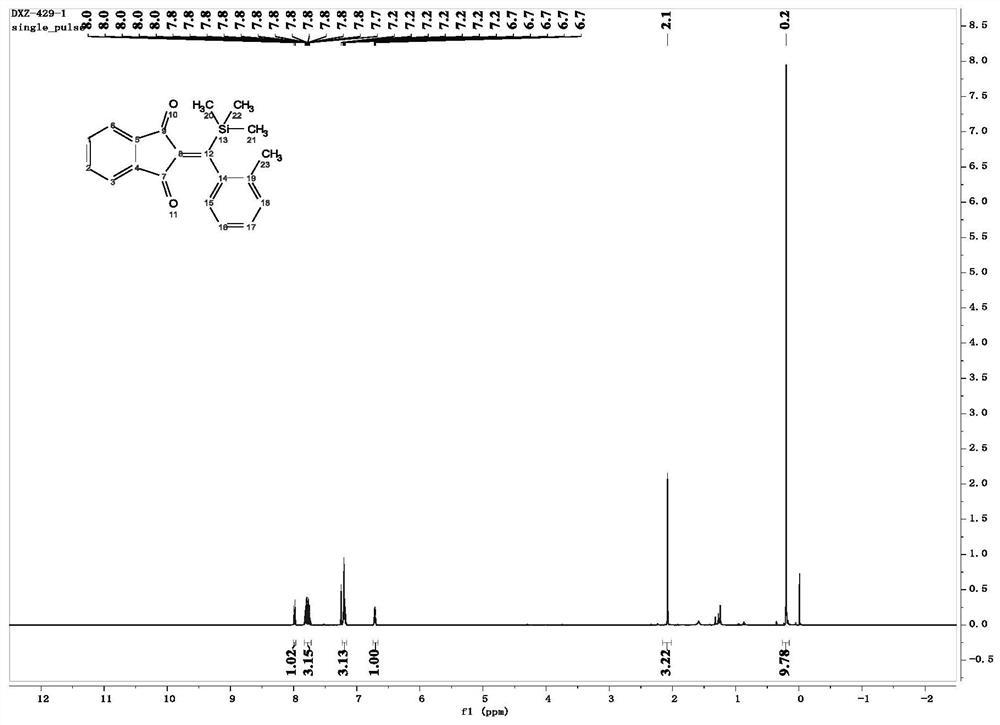
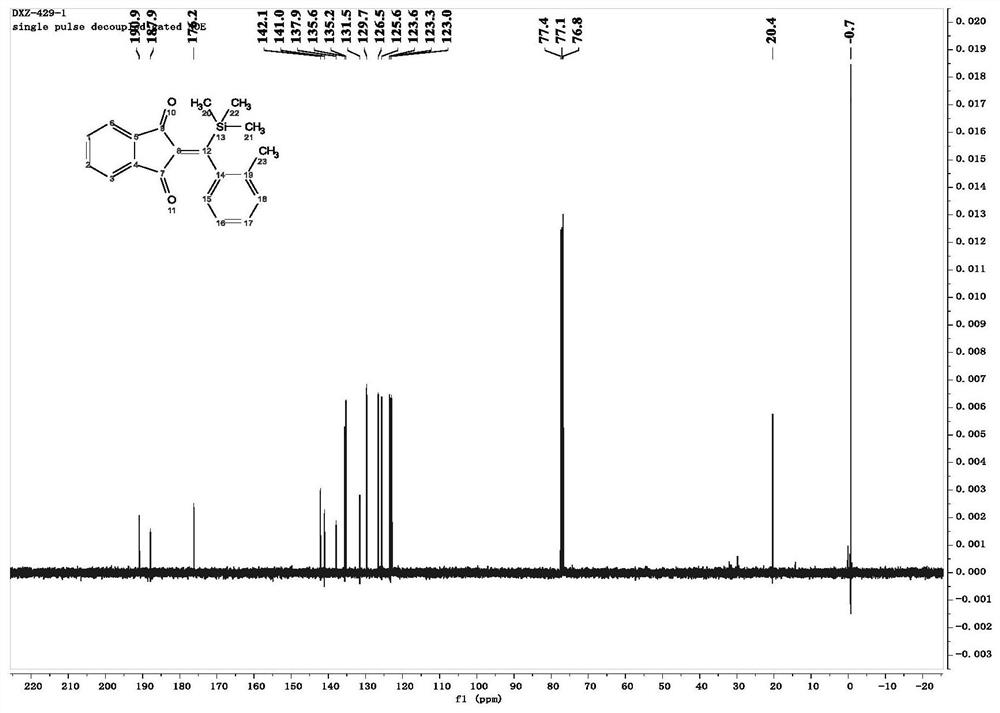
[0050] In this example, 2-(o-tolyl(trimethylsilyl)methylene)-1H-indene-1,3(2H)-dione was prepared. The specific operation is as follows:
[0051]
[0052]Under nitrogen atmosphere, 2-(3-methylsilyl)propionyl)benzonitrile (0.1mmol), 2-methylphenylboronic acid (0.25mmol), [RhOH(COD) 2 ] 2 (2.5mmol%), 1,4-dioxane (2ml) and water (0.05ml) were added to a Shrek tube equipped with a magnet. The reaction was carried out at 60° C., and the reaction was monitored using a TLC plate. When 2-(3-methylsilyl)propionyl)benzonitrile was completely reacted, the heating was stopped and the reaction was cooled to room temperature. The resulting mixture was filtered through a fritted funnel, washed with dichloromethane (10 ml, 3-5 times), and then the solvent was evaporated with a rotary evaporator under low pressure to obtain a crude product. The crude product was purified by silica gel column chromatography to obtain the desired product, 2-(o-tolyl(trimethylsilyl)methylene)-1H-indene-1,3(...
Embodiment 2
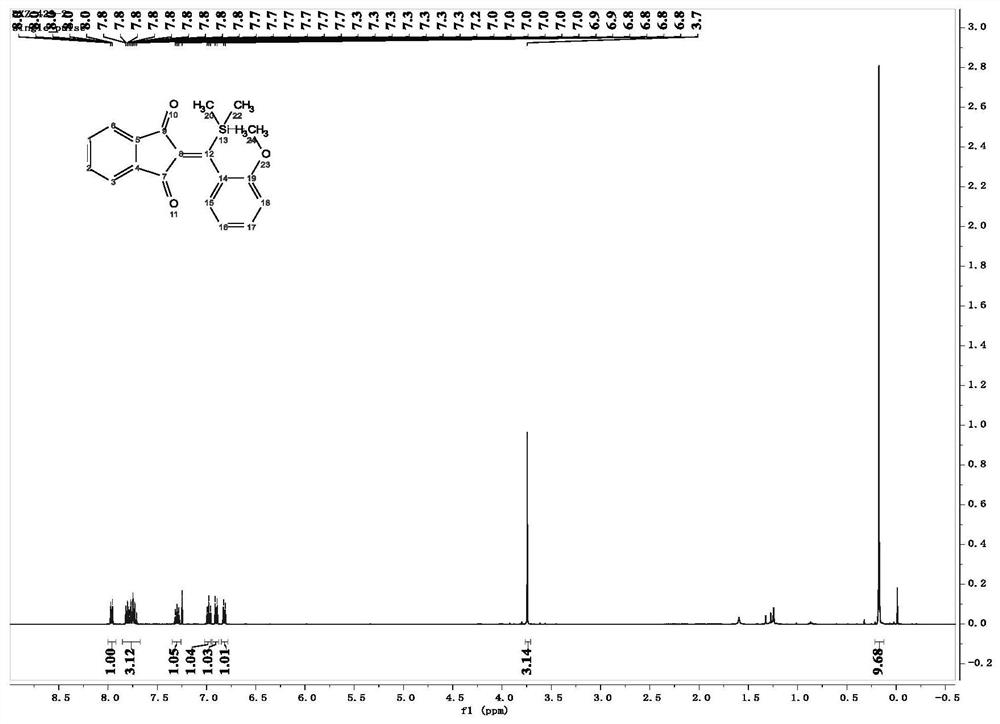
[0058] In this example, 2-((2-methoxyphenyl)(trimethylsilyl)methylene)-1H-indene-1,3(2H)-dione was prepared. The specific operation is as follows:
[0059]
[0060] Under nitrogen atmosphere, 2-(3-methylsilyl)propionyl)benzonitrile (0.1mmol), 2-methoxyphenylboronic acid (0.25mmol), [RhOH(COD) 2 ] 2 (2.5mmol%), 1,4-dioxane (2ml) and water (0.05ml) were added to a Shrek tube equipped with a magnet. The reaction was carried out at 60° C., and the reaction was monitored using a TLC plate. When 2-(3-methylsilyl)propionyl)benzonitrile was completely reacted, the heating was stopped and the reaction was cooled to room temperature. The resulting mixture was filtered through a fritted funnel, washed with dichloromethane (10 ml, 3-5 times), and then the solvent was evaporated with a rotary evaporator under low pressure to obtain a crude product. The crude product was purified by silica gel column chromatography to obtain the desired product, 2-((2-methoxyphenyl)(trimethylsilyl)met...
Embodiment 3
[0066] In this example, 2-(naphthalen-1-yl(trimethylsilyl)methylene)-1H-indene-1,3(2H)-dione was prepared. The specific operation is as follows:
[0067]
[0068] Under nitrogen atmosphere, 2-(3-methylsilyl)propionyl)benzonitrile (0.1mmol), naphthaleneboronic acid (0.25mmol), [RhOH(COD) 2 ] 2 (2.5mmol%), 1,4-dioxane (2ml) and water (0.05ml) were added to a Shrek tube equipped with a magnet. The reaction was carried out at 60° C., and the reaction was monitored using a TLC plate. When 2-(3-methylsilyl)propionyl)benzonitrile was completely reacted, the heating was stopped and the reaction was cooled to room temperature. The resulting mixture was filtered through a fritted funnel, washed with dichloromethane (10 ml, 3-5 times), and then the solvent was evaporated with a rotary evaporator under low pressure to obtain a crude product. The crude product is purified by silica gel column chromatography to obtain the desired product, 2-(naphthalen-1-yl(trimethylsilyl)methylene)-1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com