Synthesis method of dyclonine hydrochloride
A technique for the synthesis of dyclonine hydrochloride, which is applied in the direction of organic chemistry, can solve the problems of potential safety hazards, harsh storage conditions, and violent reactions, and achieve the effects of increased yield, less waste, and controllable reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 prepares dyclonine hydrochloride
[0058] (1) Preparation of 4-bromo-β-piperidinylpropiophenone hydrochloride: add 19.9g p-bromoacetophenone, 100ml ethanol, 14.6g piperidine hydrochloride (1.2eq), 8.9 g paraformaldehyde (3.0eq) and 2ml hydrochloric acid, stir and heat up to 60°C for 4 hours, stop heating, cool and crystallize at 0°C for 4 hours, filter with suction, rinse the filter cake with isopropanol, and dry it in vacuum at 60°C , 30.6kg of white solid was obtained, the HPLC purity was 97%, and the yield was 92%.
[0059] (2) Preparation of dyclonine hydrochloride: under nitrogen protection, add 183ml dimethyl sulfoxide, 16.6g 4-bromo-β-piperidinylpropiophenone hydrochloride, 1.9g cuprous iodide to a 500mL three-necked flask (0.2eq), 2.6g trans-4-hydroxy-L-proline (0.4eq), 13.8g potassium carbonate (2.0eq), 3.1g tetrabutylammonium bromide (0.19eq), 33ml acetonitrile, control kettle The inner temperature is 50°C. Quickly add 18.5g of n-butanol (5.0eq...
Embodiment 2
[0061] Embodiment 2 prepares dyclonine hydrochloride
[0062] (1) Preparation of 4-bromo-β-piperidinylpropiophenone hydrochloride: add 19.9g p-bromoacetophenone, 100ml isopropanol, 14.6g piperidine hydrochloride (1.2eq) into a 250mL three-necked flask , 8.9g of paraformaldehyde (3.0eq) and 2ml of hydrochloric acid, stirred and heated to 60°C for 4h, then stopped heating, cooled and crystallized at 0°C for 4h, filtered with suction, rinsed the filter cake with isopropanol, at 60°C After vacuum drying, 27.9kg of white solid was obtained, the HPLC purity was 90%, and the yield was 84%.
[0063] (2) Preparation of dyclonine hydrochloride: the reaction conditions were kept the same as in Example 1 to obtain 12.5 g of white solid powder with a purity of 99.2% and a yield of 77%.
[0064]It can be seen from the above results that in the reaction system of step 2) of this example, the catalyst cuprous iodide is used, and the ligand trans-4-hydroxyl-L-proline is used at the same time,...
Embodiment 3
[0073] Example 3 Structure confirmation (using the sample prepared in Example 1)
[0074] (1) Proton Nuclear Magnetic Resonance (1H-NMR)
[0075] Instrument model: BRUKER AV-400 nuclear magnetic resonance instrument, deuterated chloroform as solvent.
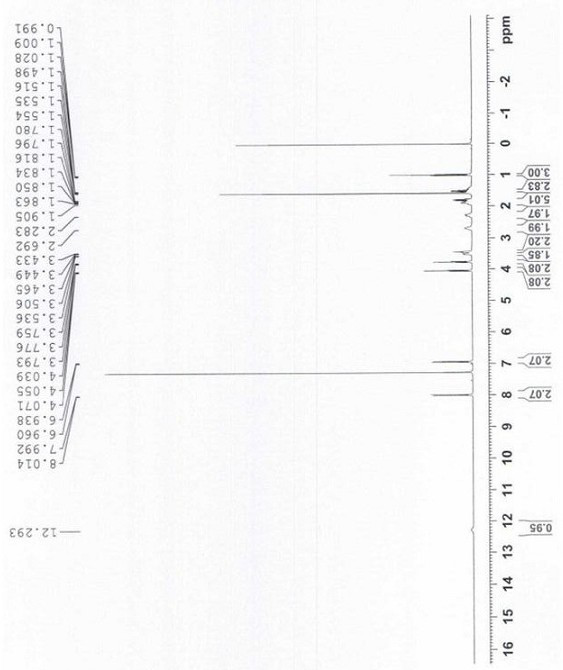
[0076] 1H-NMR (CDCl 3 , 400MHz) δ12.3(s, 1H, H-Cl), 8.0(d, 2H, Ar-H), 6.9(d, 2H, Ar-H), 4.0(t, 2H, CH 2 ), 3.7(t, 2H, CH 2 ), 3.5 (m, 2H, CH 2 ), 3.4 (m, 2H, CH 2 ), 2.7(s, 2H, CH 2 ), 2.2(m, 2H, CH 2 ), 1.9-1.0 (m, 8H, 4×CH 2 ), 1.0 (m, 3H, CH 3 )
[0077] (2) Mass spectrometry (ESI, model: Agilent 1260-6230 TOF LC-MS)
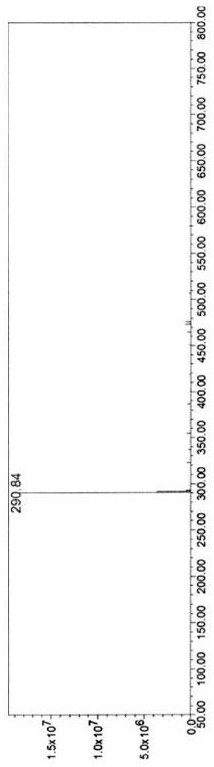
[0078] The molecular weight of dyclonine hydrochloride is 325.9, wherein the molecular weight of the free base is 289.4, the benchmark is M, and molecular ion peaks (M+1) appear in the mass spectrum + It is 290.8, consistent with the corresponding molecular weight of dyclonine hydrochloride free base.
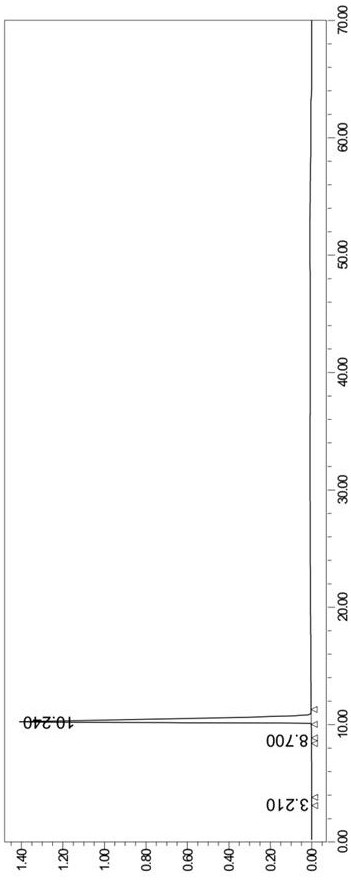
[0079] (3) HPLC purity 99.9%, determined by HPLC area normalization method, namely: chromatograph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com