Anthracene derivative-based excimer luminescent material as well as preparation method and application thereof
A technology of excimer associations and luminescent materials, which is applied in the direction of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve problems such as limiting the practical application of luminescence, and achieve mild reaction conditions and high reaction yields Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Synthesis of excimate luminescent material compound X2A based on anthracene derivatives
[0052] The synthetic route is as follows:
[0053]
[0054] Step 1): Synthesis of compound shown in formula 1
[0055] The synthetic route is as follows:
[0056]
[0057] The specific steps are as follows: 9,9-dimethylxanthene (3.00 g, 14.30 mmol) and anhydrous ferric chloride (0.12 g, 0.72 mmol) were dissolved in 15 mL of dichloromethane under an ice bath, Then dilute tert-butane chloride (3.68mL, 35.75mmol) with 15mL of dichloromethane in a constant pressure dropping funnel, add it dropwise to the above 9,9-dimethylxanthene solution under ice-cooling, and add dropwise After completion, the reaction solution was stirred at 15-35 degrees Celsius for 15 hours. After the reaction was completed, water was added to the reaction solution to quench the reaction, extracted with dichloromethane, and the organic phase was collected and dried with anhydrous sodium sulfate...
Embodiment 2
[0071] Example 2: Performance test of excimate luminescent material compound X2A based on anthracene derivatives
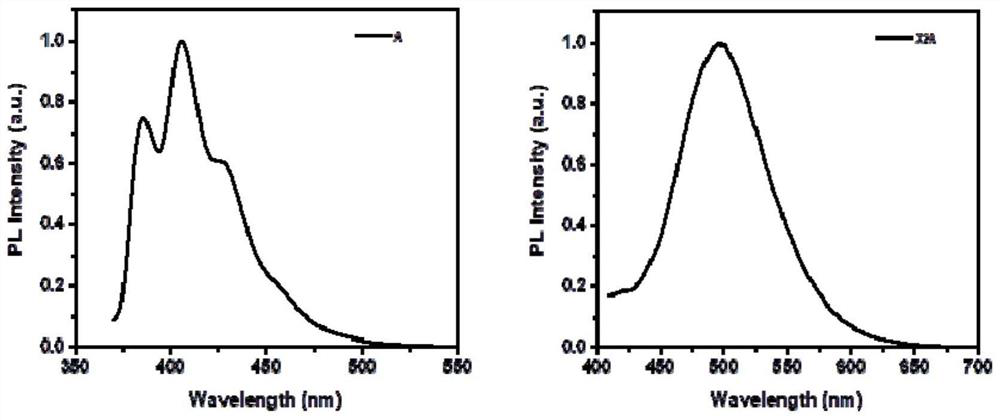
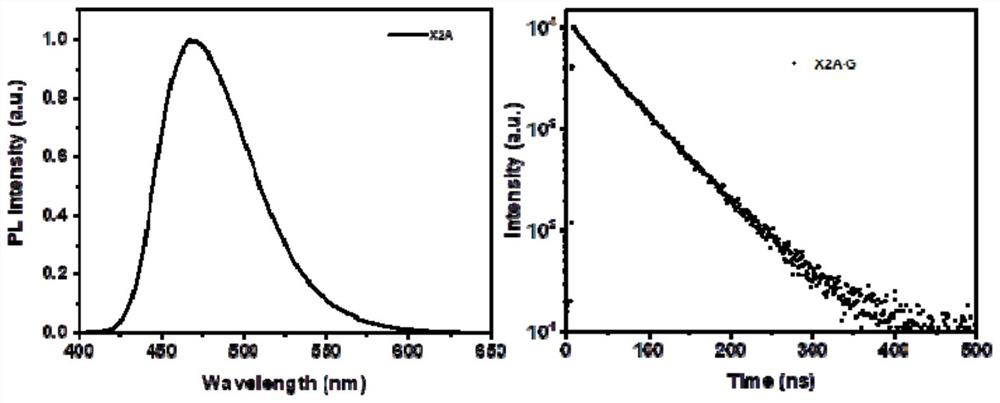
[0072] The compound represented by the formula X2A obtained in Example 1 and anthracene were respectively dissolved in an appropriate amount of dichloromethane to prepare 1×10 -5 mol / L solution, test its fluorescence emission spectrum, the results are as follows figure 2 As shown, among them, figure 2 The left is the fluorescence emission spectrum of anthracene monomer solution, figure 2 The right is the fluorescence emission spectrum of the solution of the compound represented by formula X2A. Fluorescence spectrum and fluorescence lifetime of compound single crystal shown in test formula X2A under solid state, the result is as follows image 3 As shown, among them, image 3 The left is the fluorescence spectrum of the single crystal of the compound shown in the test formula X2A under solid state, image 3The right is the fluorescence lifetime diagram of t...
Embodiment 3
[0073] Example 3: Application of excimate luminescent materials based on anthracene derivatives in the field of anti-counterfeiting
[0074] The white powder of the compound represented by formula X2A was spread on white filter paper to form the abbreviation "WHU" of Wuhan University, and the bright green "WHU" appeared on the filter paper under the irradiation of 365nm ultraviolet light. The result is as Figure 5 As shown, the results can show that the compound can be used to prepare anti-counterfeiting marks, and can be used in fields such as information storage and information transmission.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com