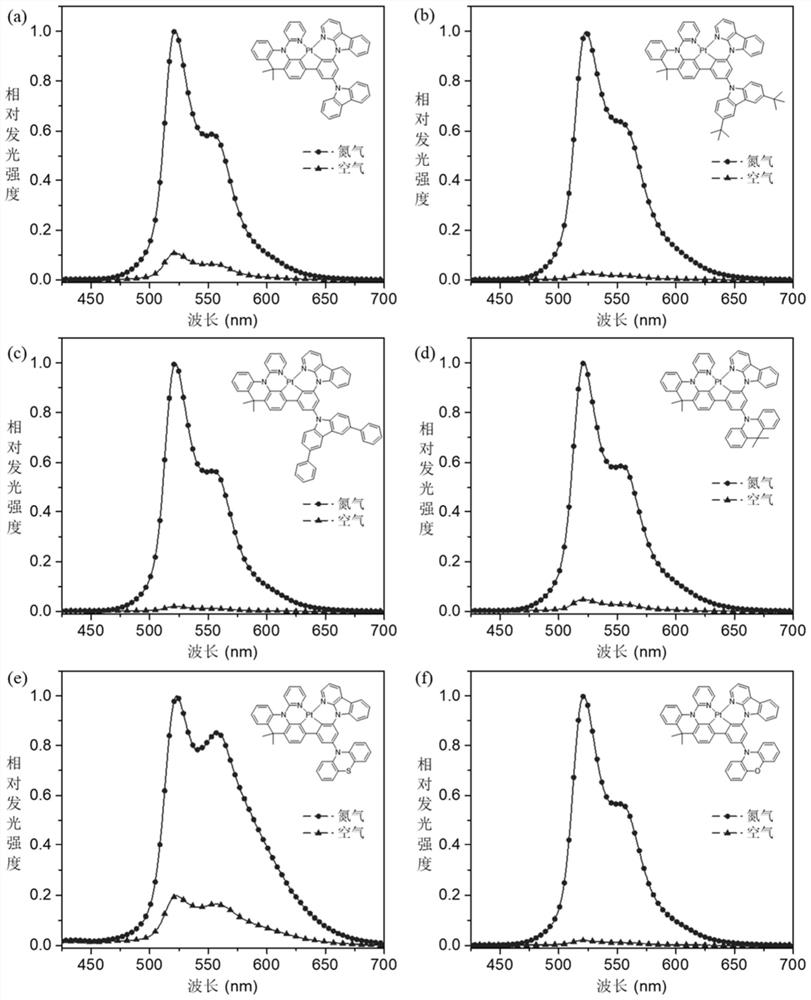
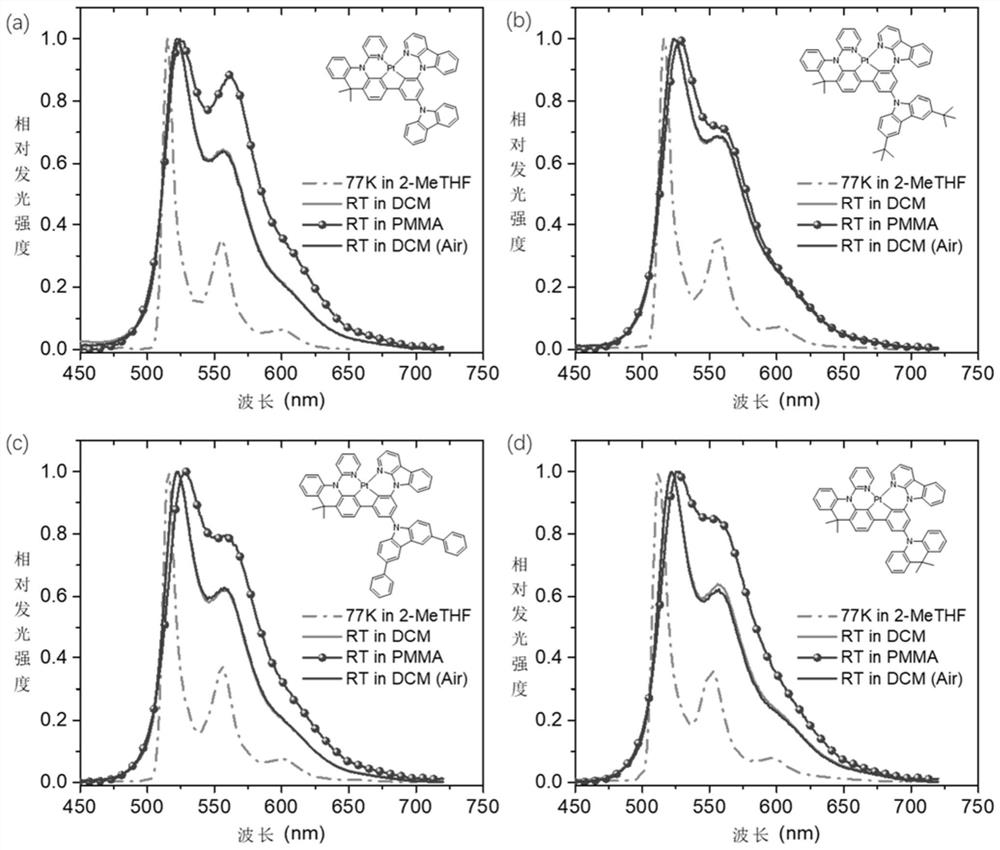
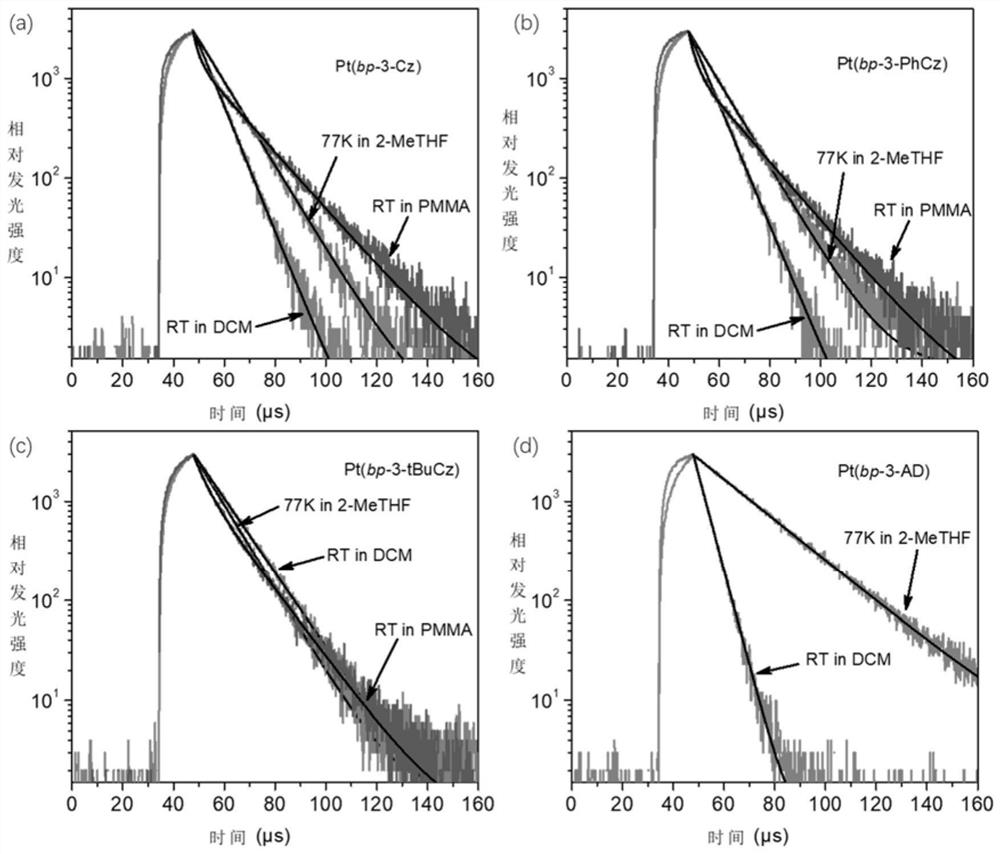
Donor-acceptor type tetradentate cyclometalated platinum or palladium complex phosphorescent material based on aromatic amine donor substituted benzene, and application thereof
An aromatic amine, phosphorescent material technology, applied in the direction of luminescent materials, palladium organic compounds, platinum group organic compounds, etc., to achieve the effect of increasing radiation rate, reducing aggregation, increasing rate and phosphorescent lotus seed efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: The synthetic route of tetracyclic metal platinum (II) complex phosphorescent material Pt (bp-3-Cz) is as follows:
[0040]
[0041]
[0042] Synthesis of intermediate (1a): Add 1,3-dibromo-5-fluorobenzene (911mg, 3.59mmol, 1.20eq), carbazole (500mg, 2.99mmol, 1.00eq ) and potassium tert-butoxide (502mg, 4.47mmol, 1.50 equiv), injected into dimethylsulfoxide (7mL). Then put it into an oil bath with magnetic stirring, raise the temperature to 140° C. and react for 15 hours, and monitor by thin-layer chromatography until the reaction of the raw materials is completed. The reaction was cooled to room temperature, diluted with ethyl acetate, and the organic phase was washed with water. After liquid separation, the organic phase was dried with anhydrous sodium sulfate. After filtration, the filtrate was distilled off under reduced pressure to remove the solvent. The obtained crude product was separated by silica gel column chromatography, eluent: petroleum ...
Embodiment 2
[0046] Example 2: The synthetic route of tetracyclic metal platinum (II) complex phosphorescent material Pt (bp-3-tBuCz) is as follows:
[0047]
[0048]
[0049] Synthesis of intermediate (2a): 9H-pyrido[2,3-b]indole (1.00g, 5.95mmol, 1.0 equivalents), cuprous iodide (1.13 g, 5.95mmol, 1.0 equivalent) and potassium phosphate (3.79g, 17.85mmol, 3.0 equivalent), the nitrogen was replaced three times, and 1,3-dibromo-5-fluorobenzene (3.02g, 11.89mmol, 2.0 equivalents), trans-1,2-cyclohexanediamine (679 mg, 5.95 mmol, 1.0 equivalents) and toluene (30 mL), put the three-necked bottle into an oil bath with magnetic stirring, and heat up to 85 ° C to react for 55 Hour. The reaction was cooled to room temperature, diluted with ethyl acetate, and the organic phase was washed with water. After liquid separation, the organic phase was dried with anhydrous sodium sulfate. After filtration, the filtrate was distilled off under reduced pressure to remove the solvent. The obtained c...
Embodiment 3
[0053] Example 3: The synthesis route of tetradentate ring metal platinum (II) complex phosphorescent material Pt (bp-3-PhCz) is as follows:
[0054]
[0055]
[0056] Synthesis of intermediate (3b): Add 2a (380mg, 1.11mmol, 1.00eq), 3,6-diphenyl-9H-carbazole (462mg, 1.45mmol, 1.30eq ) and potassium carbonate (460mg, 3.33mmol, 3.00 equiv), into dimethylsulfoxide (18mL). Then put it into an oil bath with magnetic stirring, and raise the temperature to 150° C. for 64 hours. The reaction was cooled to room temperature, diluted with ethyl acetate, and the organic phase was washed with water. After liquid separation, the organic phase was dried with anhydrous sodium sulfate. After filtration, the filtrate was distilled off under reduced pressure to remove the solvent. The obtained crude product was separated by silica gel column chromatography, eluent: petroleum ether / dichloromethane=30:1, and 360 mg of white solid was obtained with a yield of 51%. 1 H NMR (500MHz, CDCl 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com