Organic compound and application thereof
An organic compound and selected technology, applied in organic chemistry, electro-solid devices, semiconductor devices, etc., can solve the problems of device electron and hole mobility imbalance, device efficiency and life decline, and exciton formation efficiency. The effect of reducing turn-on voltage and power consumption, reducing device voltage, and improving electron mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
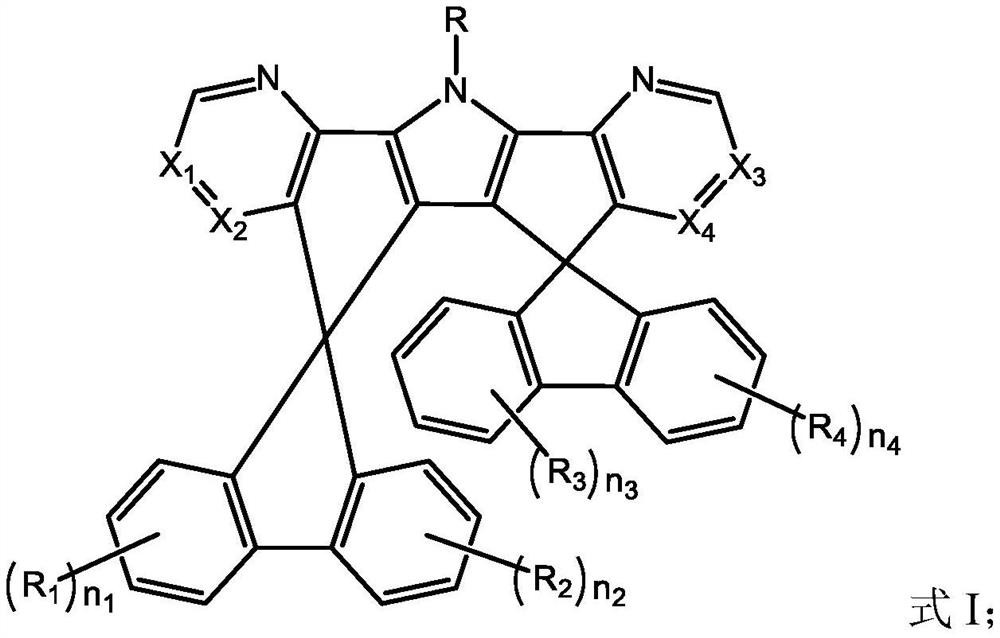
[0106] A kind of organic compound M1, the structure is as follows:
[0107]
[0108] The preparation method of this organic compound M1 comprises the steps:
[0109] (1)
[0110] In a 25 mL dry reactor (Schlenk tube), sequentially add compound A1 (0.72 g, 5 mmol), compound B1 (3.09 g, 12 mmol), Li 2 CO 3 (2.22g, 30mmol), 1,4-dioxane 15mL and diacetonitrile palladium chloride PdCl 2 (CH 3 EN) 2 (12.9 mg, 0.05 mmol). The reaction mixture was evacuated (5 times) by vacuum-argon cycle and stirred at 140 °C for 24 h. After the reaction was cooled to room temperature and concentrated, the crude mixture was purified by silica gel column chromatography to afford compound Cl.
[0111] 1 H-NMR (400MHz, CDCl 3 ): δ8.61(d, J=7.5Hz, 2H), 8.15(d, J=7.5Hz, 2H), 7.54–7.46(m, 2H), 7.45–7.39(m, 2H), 7.38–7.31( m,1H),7.09(s,2H);
[0112] 13 C-NMR (100MHz, CDCl 3):δ145.58,144.67,144.61,143.88,143.82,139.30,136.57,135.69,135.64,135.62,135.59,129.58,129.57,129.56,129.54,127.88,125...
Embodiment 2
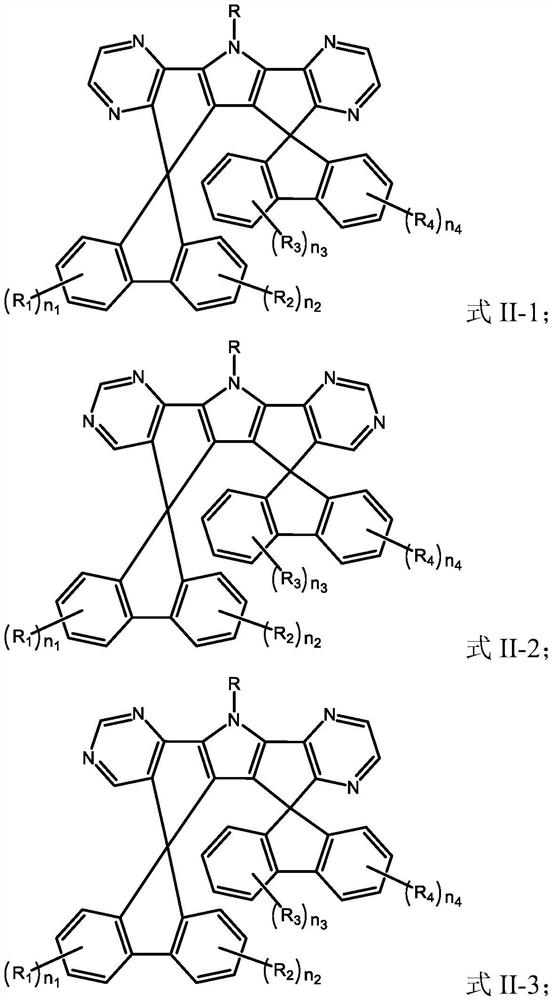
[0118] A kind of organic compound M2, the structure is as follows:
[0119]
[0120] The difference between the preparation method of the organic compound M2 and Example 1 is that the compound A1 in the step (1) is mixed with an equimolar amount of the compound A2 Replacement, other raw materials and process parameters are the same as in Example 1 to obtain the target product M2.
[0121] 1 H-NMR (400MHz, CDCl 3 ): δ8.56(q, J=15.0Hz, 4H), 7.92(s, 6H), 7.89(d, J=3.2Hz, 2H), 7.76(dt, J=4.6, 2.2Hz, 1H), 7.74 (dd, J=3.2,1.6Hz,1H),7.64(d,J=3.4Hz,2H),7.61(d,J=3.4Hz,2H),7.53–7.39(m,3H),7.34(td, J=14.9, 3.4Hz, 4H), 7.24(td, J=14.8, 3.3Hz, 4H).
Embodiment 3
[0123] A kind of organic compound M3, the structure is as follows:
[0124]
[0125] The difference between the preparation method of the organic compound M3 and Example 1 is that the compound A1 in the step (1) is mixed with an equimolar amount of the compound A3 Replacement, other raw materials and process parameters are the same as in Example 1 to obtain the target product M3.
[0126] 1 H-NMR (400MHz, CDCl 3 ): δ8.58(d, J=7.5Hz, 2H), 8.54(d, J=7.5Hz, 2H), 8.20(s, 2H), 7.90(dd, J=7.4, 1.5Hz, 4H), 7.61 (dd, J=7.4, 1.5Hz, 4H), 7.34 (td, J=7.5, 1.5Hz, 4H), 7.24 (td, J=7.5, 1.4Hz, 4H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com