Optical selective process synthesis method of (5R)-5-hydroxytriptolide
A compound and inert solvent technology, applied in the field of optical selective process synthesis of -5-hydroxytriptolide, can solve the problem of high labor protection and environmental protection requirements, high requirements for production workshops and equipment, and the impossibility of mass production Continuous acquisition and other issues to achieve the effects of avoiding column chromatography separation, low protection requirements, and reduced toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] To a three-neck flask was added 50g (1 eq) Triptonide (compound of formula II) and dioxane 500mL, followed by addition of selenium dioxide 124g (8eq), heated to reflux and stirred until the reaction was complete. After bringing back to room temperature and filtered to remove insolubles. The filtrate was concentrated, water was added and extracted with dichloromethane. The organic layer was washed with water continues, and concentrated to give crude compound of formula III, as a pale yellow solid, yield 88%. The crude product was used without purification in the next reaction.
Embodiment 2
[0044]
[0045] (Compound of formula II) to a three-neck flask was added 3g (1 eq) and dimethyl sulfoxide Triptonide 20mL, followed by addition of selenium dioxide 3.7g (4eq), was heated to 120 to 100 [deg.] C and stirred until the reaction was complete. After bringing back to room temperature and filtered to remove insolubles. The filtrate was concentrated, extracted with ethyl acetate and water. The organic layer was washed with water continues, and concentrated to give crude compound of formula III, 80% yield. The crude product was used without purification in the next reaction.
Embodiment 3
[0047]
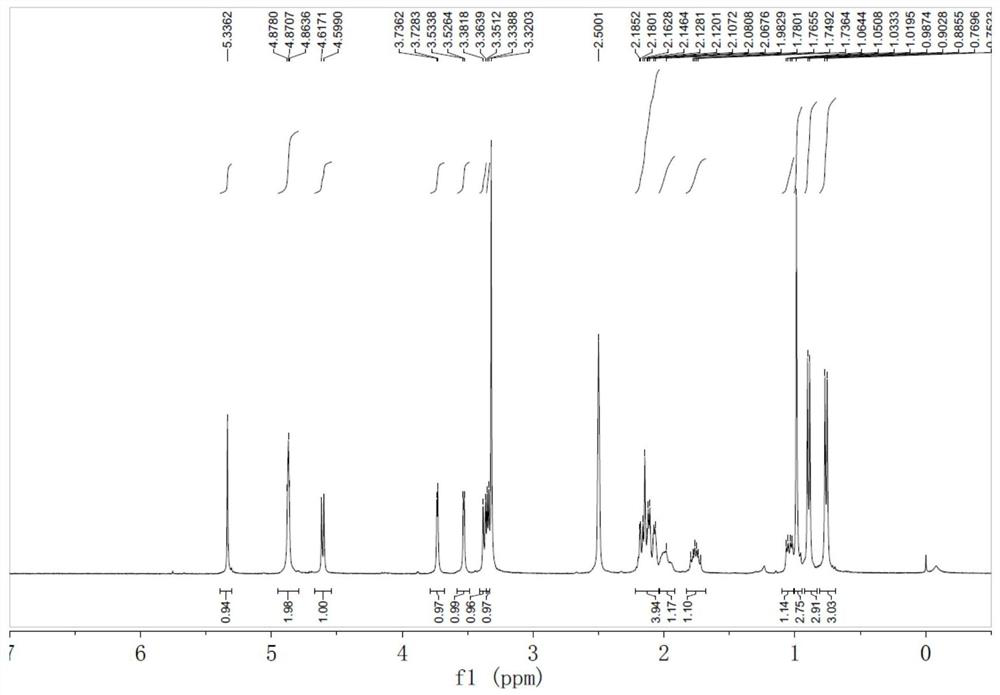
[0048]17 g of formula III compound was added to the three flask and 170 ml of anhydrous tetrahydrofuran, and dissolved to -60 to 50 ° C after dissolving. This temperature was maintained and 100 ml (2.2 eq) of 1M triamoliol hydride tetrahydrofuran was added dropwise. After stirring until the reaction was complete, raised to 0-10 ° C. This temperature was maintained at 170 mL of water, then the pH was adjusted with a 5% hydrochloric acid solution. After the aqueous phase was extracted twice with ethyl acetate, the organic phase was combined, filtered through 50 g of silica gel, the filter cake was sufficiently collected with ethyl acetate, and the filtrate was concentrated to give a crude product. The crude filtered ethanol was purified and purified, and the compound of formula I, white powder was obtained, and the yield was 83%. 1 H NMR (DMSO-D 6 400MHz): δ = 5.34 (S, 1H), 4.60 (S, 2H), 4.60 (D, J = 7.2 Hz, 1H), 3.73 (D, J = 3.2Hz, 1H), 3.53 (D, J = 2.8Hz, 1H), 3.37 (D, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com