Method for preparing series of sucrose esters in green solvent
A green solvent and sucrose ester technology, applied in the field of sucrose ester manufacturing, can solve problems such as toxic residues, food safety hazards, and environmental pollution, and achieve the effects of preventing caramelization, excellent antibacterial properties, and high economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
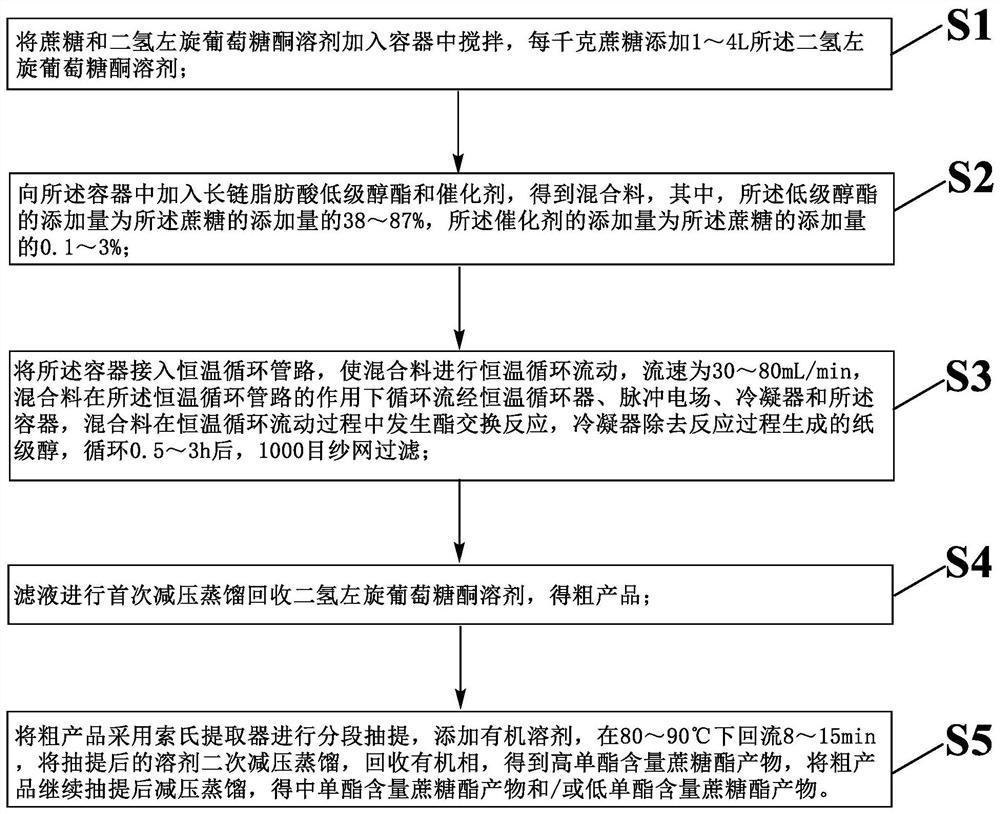
[0045] A kind of method that prepares series sucrose ester in green solvent, carries out as follows:
[0046] Step S1: Put 1Kg of sucrose in a container and add 3.8L of dihydro-levoglucosone solvent, and stir at 60-65°C for 45 minutes;
[0047] Step S2: Add 0.58Kg industrial grade methyl stearate, 0.01Kg potassium carbonate, 5g of ZnTAC to the container 24 , to get the mixture;
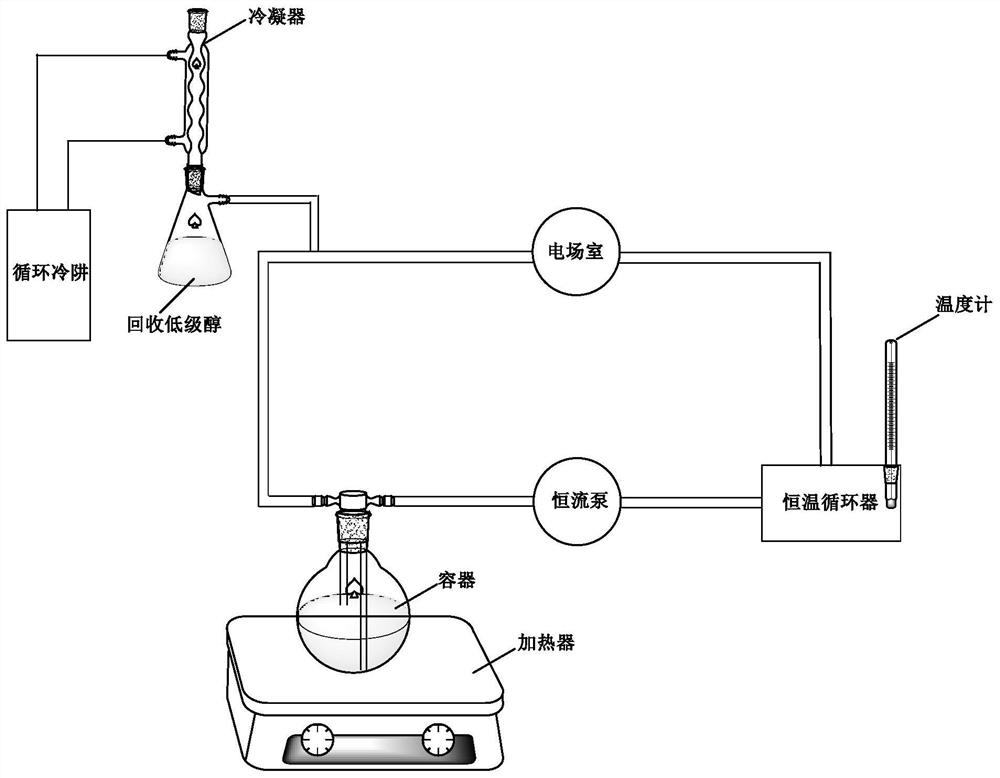
[0048] Step S3: Connect the container to a constant temperature circulation pipeline, so that the mixture is circulated and mixed. The pressure in the constant temperature circulation pipeline is 10kPa, and the flow rate is 65mL / min. The mixture is under the action of the constant temperature circulation pipeline. The circulation flows through the constant temperature circulator, the pulse electric field, the condenser and the container, and the mixed material undergoes transesterification reaction in the constant temperature circulation pipeline. The constant temperature circulation pipeline include...
Embodiment 2
[0060] A kind of method that prepares series sucrose ester in green solvent, carries out as follows:
[0061] Step S1: Put 1Kg of sucrose in a container and add 3.5L of dihydro-levoglucosone solvent, and stir at 60-65°C for 45 minutes;
[0062] Step S2: Add 0.87Kg industrial grade methyl stearate, 0.01Kg potassium carbonate, 3g CaO·γ-Fe to the container 2 o 3 , to get the mixture;
[0063]Step S3: Connect the container to a constant temperature circulation pipeline to circulate and mix the mixture. The pressure in the constant temperature circulation pipeline is 8kPa, the flow rate is 58mL / min, and the mixture circulates under the action of the constant temperature circulation pipeline. Flowing through constant temperature circulator, pulse electric field, condenser and described container, mixed material carries out transesterification reaction in described constant temperature circulation pipeline, and described constant temperature circulation pipeline comprises sequentia...
Embodiment 3
[0075] This example is the same as the operation steps in Example 1, but focuses on the feasibility of verifying the recovery of by-products and solvents, the specific differences are as follows:
[0076] (1) The methyl stearate used in step S2 comes from the methanol collected in step S3 of Example 1 carried out several times, and is prepared by sulfuric acid catalyzed esterification with stearic acid; The reclaimed dihydro-L-glucosone solvent of step S4 of embodiment 1; ZnTAC used 24 From the solid catalyst recovered in step S3 of Example 1.
[0077] (2) The organic solvent used in step S5 comes from the ethanol recovered from the organic phase of step S5 in Example 1.
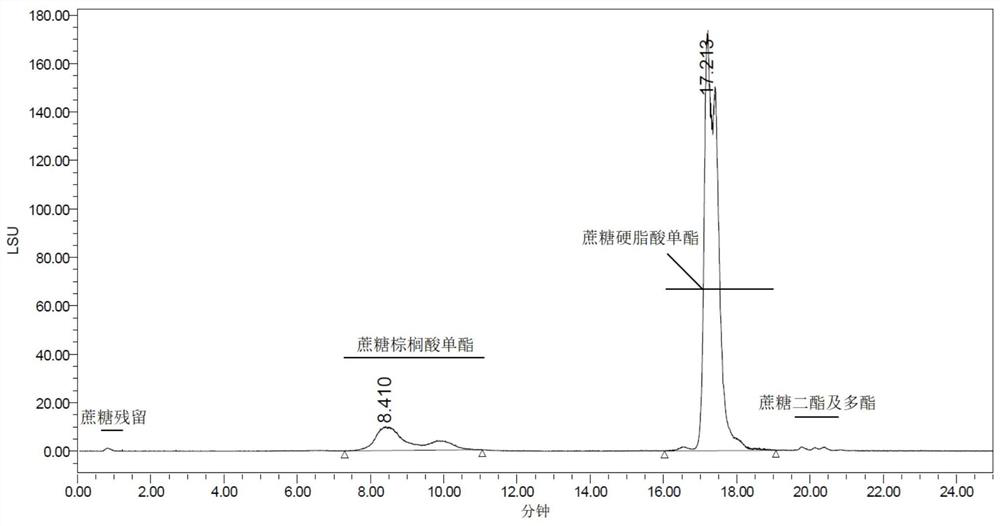
[0078] According to the method for product quality inspection in embodiment 1, the various quality indicators of sucrose ester products 5 and 6 obtained are detected, and the indicators of the series of sucrose ester products 5 and 6 are the same as those of the series of sucrose ester products 1 and 6 obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com