Somatostatin analogue and application thereof
A technology of somatostatin and analogs, applied in the field of biomedicine, can solve the problems of low tumor specificity and missed diagnosis, and achieve the effect of good targeting, short labeling time and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
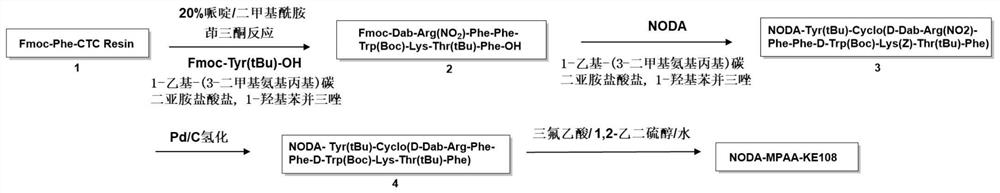
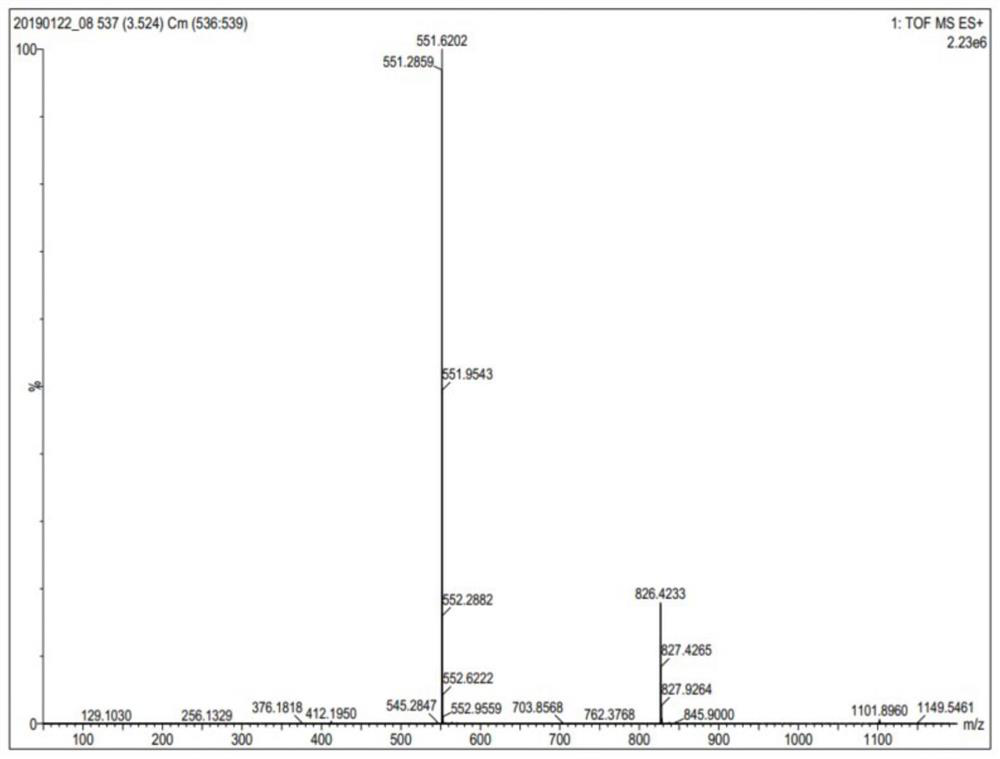
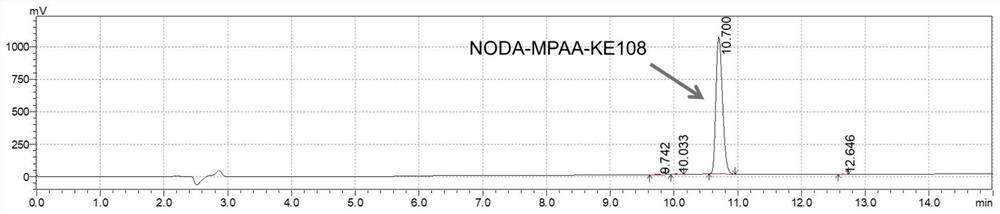
[0036] The following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention. Unless otherwise specified, the technical means used in the examples are conventional means well known to those skilled in the art, and the raw materials used are all commercially available products. Example 1 Preparation of somatostatin analogue NODA-MPAA-KE108 and its labeled compound
[0037] The structure of the somatostatin analog provided in this embodiment is shown in formula I):
[0038]
[0039] Wherein, R is a bifunctional chelating group, and R1 is a radionuclide.
[0040] The bifunctional chelating group is -DTPA, -NOTA, -p-SCN-Bn-NOTA, -DOTA or -MPAA-NODA and the like.
[0041] R1 can be selected from 18 F. 64 Cu, 68 At least one of radionuclides such as Ga.
[0042] 1, the preparation method of somatostatin analog is as follows:
[0043] (1) Synthesis of NODA-MPAA-KE108 polypeptide (see figure 1 )
[0044] ① Res...
Embodiment 2
[0066] Example 2 Affinity test with SSTR
[0067] Affinity studies of somatostatin analogs with each subtype of SSTR were tested using surface plasmon resonance (SPR). Ultrafiltration centrifugation was used to detris the five subtype receptors of SSTR1-5, and a dextran chip was used. Each chip was set with a reference channel, and the other channels were used to couple SSTR proteins. The SSTR protein was formulated in pH 4.5 sodium acetate buffer (10 mM) at a concentration of 200 μg / mL for coupling to the chip. The somatostatin analog (1mM) was sequentially diluted with phosphonate Tween solution (PBST) into 10 gradients (0.1-100μM), and a blank PBST was added every 5 gradients, starting from the low concentration. machine test. The test results are shown in Table 1.
[0068] Table 1 Affinity constants K of somatostatin and its analogs with five protein isoforms d Value (M)
[0069]
[0070] Note: For SST14 and SST28, see Gao J, Tong H, Huang Z, et al. Affinity analys...
Embodiment 3
[0072] Example 3 In vitro stability studies
[0073] Take 100 μL of [Al 18 F]NODA-MPAA-KE108 was added to physiological saline solution (pH7.4), incubated at room temperature for 0.5h, 1h, and 2h, and then detected by HPLC. Similarly, 100 μL of [Al 18 F]NODA-MPAA-KE108 was added to 1.9 mL of 10% fetal bovine serum, incubated at 37°C for 0.5 h, 1 h, and 2 h, 400 μL of the above serum was added to 100 μL of acetonitrile, mixed, vortexed, centrifuged through the membrane, and then tested by HPLC. For test results see Image 6 (a and b), the results show that [Al 18 F]NODA-MPAA-KE108 maintained good stability in normal saline (a) and 10% fetal bovine serum (b), and the radiochemical purity was >95%.
[0074] Example 4 PET imaging of tumor-bearing mice
[0075] AR42J tumor-bearing mice (BALB / c nude mice, male, about 20 g) with a tumor diameter of about 0.5-1 cm were injected through the tail vein [Al 18 F]NODA-MPAA-KE108 (3.7MBq / 100μL·mice), PET imaging was performed at 0.5h a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com