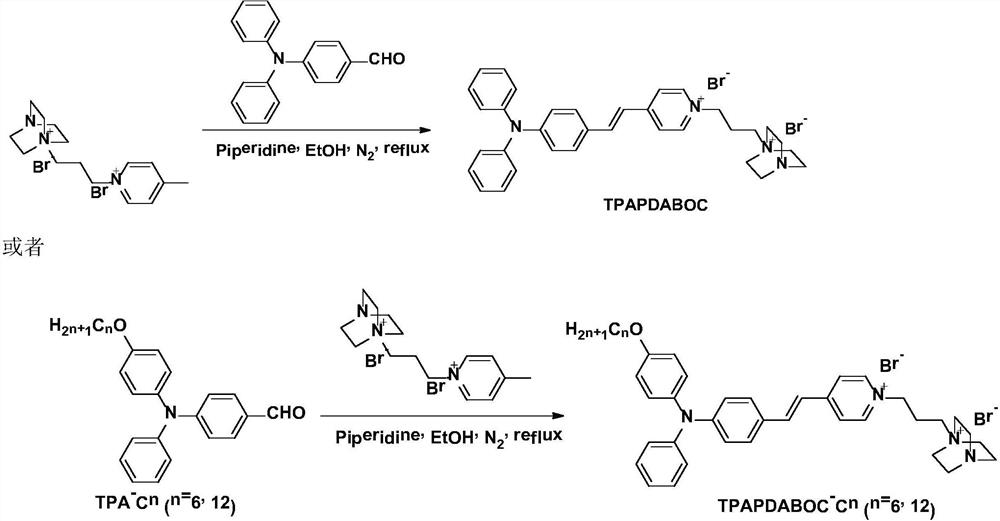
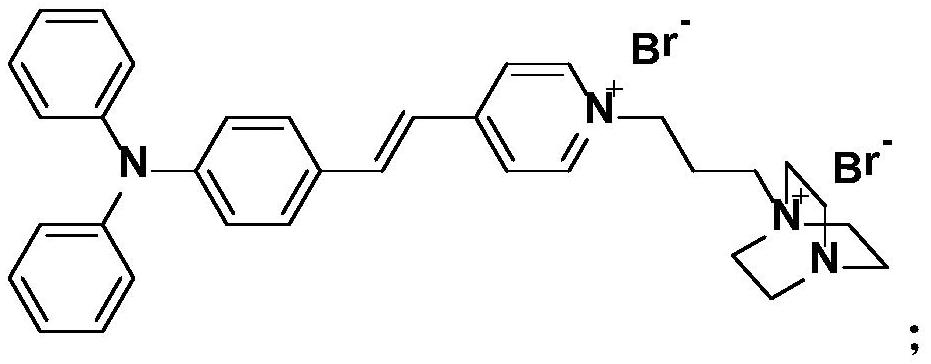
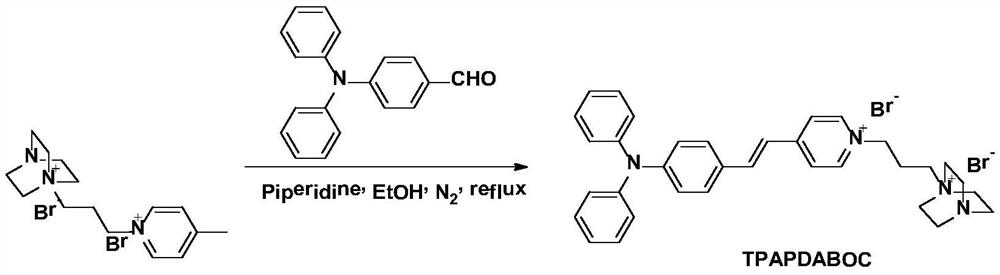
Near-infrared aggregation-induced emission membrane probe molecule, and preparation method and application thereof
A technology of aggregation-induced luminescence and probe molecules, which is applied in the field of optical probes, can solve the problems of optical probe quenching, inability to trace fluorescence, and fluorescence quenching, etc., and achieve increased residence time, good water solubility, and fast dyeing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the synthesis of single charge big steric hindrance salt
[0044] Dissolve 1,4-diazidobicyclo[2.2.2]octane (2.24g, 20mmol) in 100mL acetone, add 1,3-dibromopropane (2.02mL, 20mmol), stir overnight at room temperature, a white solid precipitates . Pour off the liquid on the upper part of the precipitation, wash with acetone by decantation, repeat 3 to 4 times to remove unreacted raw materials, spin dry the solid under reduced pressure and vacuum at 40°C, and seal it for storage.
Embodiment 2
[0045] Embodiment 2, the synthesis of two charge big steric hindrance salt
[0046] Dissolve single-charge bulky hindered salt (1.57g, 5mmol) in 50mL ethanol, vacuumize, fill with nitrogen, add 4-methylpyridine (4.866mL, 50mmol), heat to reflux at 80°C for 48h. The solution gradually darkened from clear and transparent, and finally turned brownish yellow. After the reaction, spin the ethanol to dryness, add a large amount of acetone and a small amount of ethanol (used to remove the raw material salt) mixed solution to the crude product, heat and reflux for 1 hour, pour off the supernatant after cooling, repeat the operation 3 to 4 times, use a developing Agent EA:PE=1:5 to climb the plate, judge whether the 4-picoline is completely removed, after the removal, the solid is dried under reduced pressure and vacuum at 40°C, and then sealed and stored to obtain a brown-yellow solid.
Embodiment 3
[0047] The synthesis of embodiment 3,4-hexyloxydiphenylamine
[0048] Add cesium carbonate (7.78g, 23.88mmol) to 30mL of dry DMF, vacuumize, fill with nitrogen, add 4-hydroxydiphenylamine (2.65g, 14.32mmol), and add hexyl bromide (1.68mL, 11.94 mmol), reacted at 100°C for 6h. After the reaction, the solution was brownish black, extracted with ethyl acetate, the organic layer was washed 3 times with water and dried with anhydrous sodium sulfate, the sample was mixed with silica gel powder and spin-dried, EA:PE=1:30 was passed through the column, and a white solid was obtained. Visibly fluorescent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com