A kind of preparation technology of cefuroxime sodium for injection
A technique for preparing cefuroxime sodium and its preparation process, which is applied in the field of preparation process of cefuroxime sodium for injection, can solve problems such as easy residual solvent, improper operation, and influence on drug quality and safety, and achieve reduction of residual water and promotion of precipitation , Improve the crystallization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
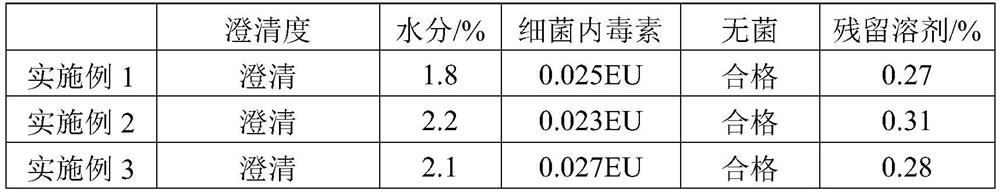
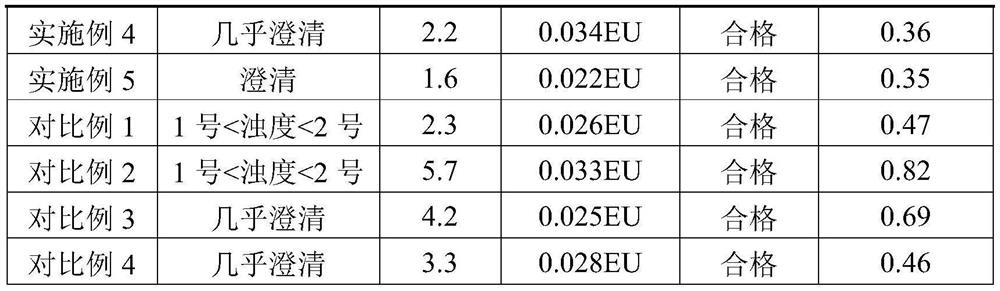
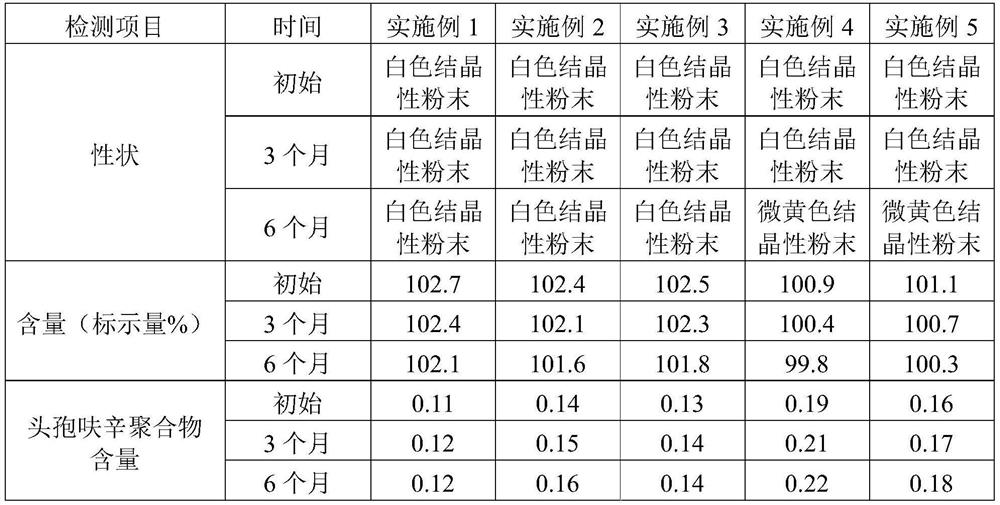
Examples
Embodiment 1
[0026] A preparation process of cefuroxime sodium for injection, comprising the following steps:
[0027] S1. Preparation of phosphate buffered saline solution: mix disodium hydrogen phosphate solution and sodium dihydrogen phosphate solution, adjust the pH to 6.8, aseptically process, and prepare a phosphate buffer solution with a concentration of 0.2 mol / L;
[0028] S2. Preparation of resin: pulverize the starch to 350 mesh, dissolve the starch with water, then add poly-N-isopropylacrylphthalamide and 0.75 times the weight of starch by adding poly-N-isopropylacrylphthalamide and 0.75 times the weight of the starch. The temperature is controlled at 190°C, the pressure is controlled at 0.55MPa, and after 40min of treatment, it is returned to the normal pressure level, filtered, and the filter cake is granulated. ;
[0029] S3. Decolorization: add cefuroxime sodium to the phosphate buffered saline solution of step S1, stir until completely dissolved, add activated carbon 10 ti...
Embodiment 2
[0032] A preparation process of cefuroxime sodium for injection, comprising the following steps:
[0033] S1. Preparation of phosphate buffered saline solution: mix disodium hydrogen phosphate solution and sodium dihydrogen phosphate solution, adjust the pH to 7.0, aseptically process, and prepare a phosphate buffer solution with a concentration of 0.2 mol / L;
[0034] S2. Preparation of resin: pulverize the starch to 200 mesh, dissolve the starch with water, then add poly-N-isopropylacrylphthalamide and 0.83 times the weight of starch by adding poly-N-isopropylacrylphthalamide and 0.83 times the weight of the starch. The temperature is controlled at 195°C, and the pressure is controlled at 0.5MPa. After treatment for 1 hour, it is restored to the normal pressure level, filtered, and the filter cake is granulated. After soaking the granules in a phosphate buffer solution for 10 minutes, drying is performed to obtain the resin;
[0035] S3. Decolorization: add cefuroxime sodium ...
Embodiment 3
[0038] A preparation process of cefuroxime sodium for injection, comprising the following steps:
[0039] S1. Preparation of phosphate buffered saline solution: mix disodium hydrogen phosphate solution and sodium dihydrogen phosphate solution, adjust the pH to 6.5, aseptically process, and prepare a phosphate buffer solution with a concentration of 0.2 mol / L;
[0040] S2. Preparation of resin: pulverize the starch to 400 mesh, dissolve the starch with water, then add poly-N-isopropylacrylphthalamide and 0.7 times the weight of the starch by adding poly-N-isopropylacrylphthalamide and 0.7 times the weight of the starch. The temperature is controlled at 185°C, and the pressure is controlled at 0.65MPa. After treatment for 0.5h, return to the normal pressure level, filter, take the filter cake and granulate, soak the particles in phosphate buffer solution for 30min, and then dry to obtain the resin;
[0041] S3. Decolorization: add cefuroxime sodium to the phosphate buffered sali...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com