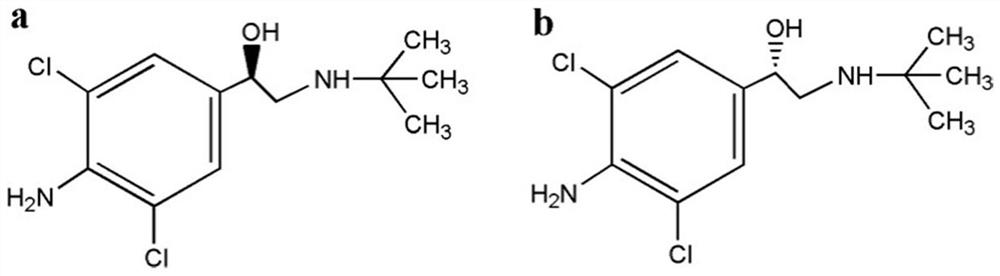
Clenbuterol enantiomer resolution and determination method based on ultra performance convergence chromatography technology
A technique for efficient convergence chromatography and enantiomeric separation, applied in the field of high-efficiency detection of clenbuterol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
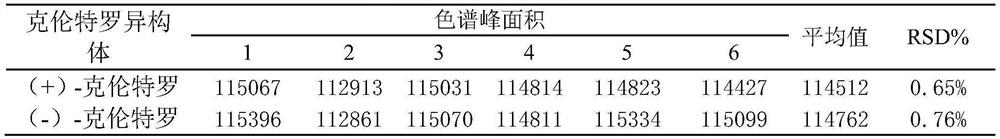
[0024] 1 Experimental part
[0025] 1.1 Instruments, materials and reagents
[0026] Acquity ultra-high efficiency convergence chromatograph (U.S. Waters company, with PDA diode array detector); AE260 electronic balance (Swiss Mettler company); R215 rotary evaporator (Switzerland Buchi company); ELGA CLXXXUVM2 ultrapure water purification system (UK Elga company); MS2 vortex mixer (Shanghai Medical University Instrument Factory); N-EVAP TM 111 Nitrogen Blowing Apparatus (Tokyo Physical and Chemical Company, Japan).
[0027] Acetonitrile, methanol, formic acid, ethanol, isopropanol, n-heptane (chromatographically pure, Scharlau, Spain); ammonium acetate, ammonia water (excellent pure); water is ultrapure water; high-purity carbon dioxide (99.999%); other experiments All reagents used were of analytical grade unless otherwise specified.
[0028]Chiral separation column: Acquity Trefoil CEL2 (3.0mm×150mm, 2.5μm, filled with cellulose-tris(3-chloro-4-methylphenylcarbamate)), A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com