Abiraterone acetate pharmaceutical composition, preparation, preparation method and application thereof
A technology of abiraterone acetate and its composition, which is applied in the direction of drug combination, drug delivery, and pharmaceutical formulation, which can solve the problems of slow drug dissolution rate and absorption, high risk of adverse reactions, and poor patient compliance, and achieve treatment costs. Effect of reducing, enriching drugs and pathways, increasing bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
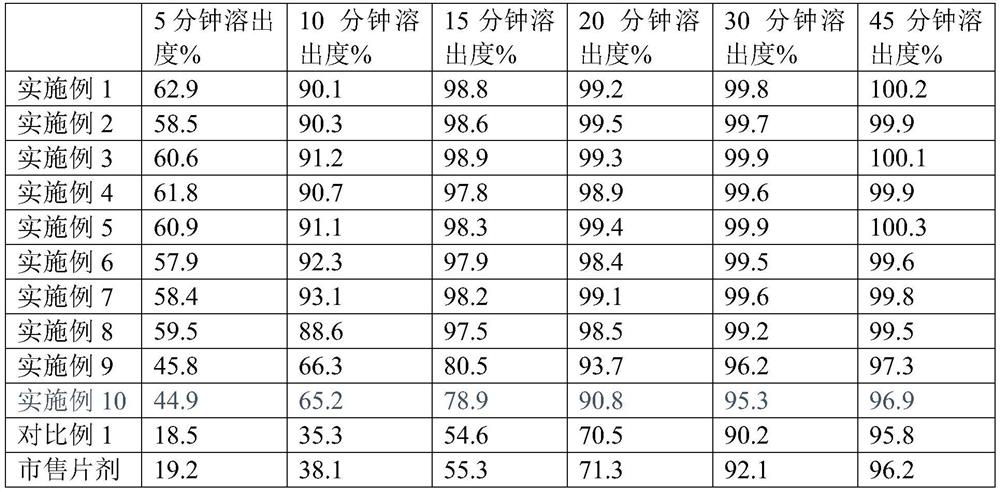
Embodiment 1
[0081] Embodiment 1: Abiraterone acetate tablet preparation
[0082] (1) 69 parts of lactose and 10 parts of sodium starch glycolate are premixed to obtain a premix;
[0083] (2) Mix 10 parts of povidone and 100 parts of water to obtain an aqueous binder solution, and use a fluidized bed to prepare the premix and the aqueous binder solution into matrix particles;
[0084] (3) 10 parts of abiraterone acetate are dissolved in 300 parts of chloroform, sprayed onto the matrix particles, dried,
[0085] (4) granulate, add 1 part of magnesium stearate, mix, tabletting, obtain abiraterone acetate tablet.
Embodiment 2
[0086] Embodiment 2: Abiraterone acetate tablet preparation
[0087] (1) 50 parts of lactose and 9 parts of sodium starch glycolate are premixed to obtain a premix;
[0088] (2) Mix 20 parts of povidone and 200 parts of water to obtain an aqueous binder solution, and use a fluidized bed to prepare the premix and the aqueous binder solution into matrix particles;
[0089] (3) 20 parts of abiraterone acetate are dissolved in 600 parts of chloroform, sprayed onto the matrix particles, dried,
[0090] (4) granulate, add 1 part of magnesium stearate, mix, tabletting, obtain abiraterone acetate tablet.
Embodiment 3
[0091]Embodiment 3: preparation of abiraterone acetate tablet
[0092] (1) 50 parts of lactose, 10 parts of microcrystalline cellulose and 9.5 parts of sodium carboxymethyl starch are premixed to obtain a premix;
[0093] (2) Mix 20 parts of starch and 200 parts of water to obtain an aqueous binder solution, and use a fluidized bed to prepare the premix and the aqueous binder solution into matrix granules;
[0094] (3) 10 parts of abiraterone acetate are dissolved in 350 parts of chloroform and 350 parts of ethanol, sprayed onto the matrix particles, dried,
[0095] (4) granulate, add 0.05 part of magnesium stearate, mix, tabletting, obtain abiraterone acetate tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com