Preparation method of boscalid
A technology of boscalid and formamide, applied in the field of compound synthesis, can solve problems such as high cost and environmental harm, and achieve the effects of low cost, reduced environmental damage, and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] One of the objects of the present invention is to provide a preparation method of boscalid, characterized in that, comprising the following steps:
[0070] 1) making biphenyl-2-formic acid and an acyl chloride reagent carry out acyl chloride reaction to obtain biphenyl-2-formyl chloride;
[0071] 2) making the biphenyl-2-formyl chloride obtained in step 1) undergo an amidation reaction with an amidating reagent to obtain biphenyl-2-formamide;
[0072] 3) making the biphenyl-2-carboxamide obtained in step 1) react with chlorine gas in the presence of a catalyst to obtain 4'-chloro-2-biphenylcarboxamide;
[0073] 4) the 4'-chloro-2-biphenylcarboxamide obtained in step 3) is subjected to a Hoffman rearrangement reaction to obtain 4'-chloro-2-aminobiphenyl;
[0074] 5) The resulting 4'-chloro-2-aminobiphenyl is condensed with 2-chloronicotinyl chloride in the presence of an inorganic base to obtain boscalid.
[0075] Each step of the preparation method of the present inve...
Embodiment 1
[0129] Embodiment 1: the synthesis of biphenyl-2-formic acid
[0130] At room temperature, 9-fluorenone (750 grams), potassium hydroxide (375 grams) and toluene (3700 milliliters) of 90% purity were added in the reaction flask, and the reaction flask was placed in a thermal oil bath and heated to about 105° C. The internal temperature suddenly began to rise violently automatically and rose to 124°C in about 20 seconds, and a large amount of foam was formed. The temperature was maintained at 120°C, the toluene was refluxed, and the reaction was carried out for 1 hour. After the completion of the reaction was monitored by thin-layer chromatography (TLC), the reaction system was cooled to about 50°C, water (4000 ml) was added to the reaction system, the phases were extracted and separated, the aqueous phase was collected, adjusted to PH≈3 with hydrochloric acid, and precipitated A large amount of white solid was centrifuged and dried to obtain biphenyl-2-carboxylic acid (819 g, p...
Embodiment 2
[0131] Embodiment 2: the synthesis of biphenyl-2-formic acid
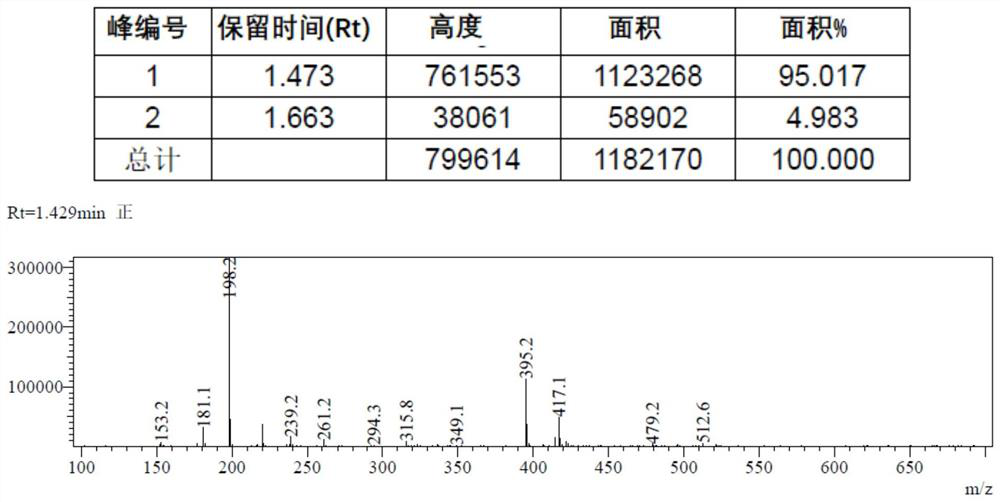
[0132] 9-Fluorenone (750 g) was dissolved in toluene (1800 mL) at room temperature. Add potassium hydroxide (375 grams) and toluene (1800 milliliters) with a purity of 90% into the reaction flask at room temperature, heat it to about 105° C., and add the above-mentioned 9-fluorenone dropwise to the reaction flask with a constant pressure dropping funnel. Toluene solution, the dropwise addition time is about 20 minutes. Keep the temperature at 105°C and react for 9h. After TLC monitors that the reaction is over, cool the reaction system to about 50°C, add water (4000 ml) to the reaction system, extract and separate the phases, collect the water phase, adjust to PH ≈ 3 with hydrochloric acid, and precipitate a large amount of white solid. After separation and drying, biphenyl-2-carboxylic acid (813 g, purity 99%, yield 98.5%) was obtained. MS: [M - =197.1]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com