Preparation method of carbazole polyaromatic piperidine anion exchange membrane
An anion exchange membrane and polyaromatic piperidine technology, which is applied in the field of preparation of carbazole polyaromatic piperidine anion exchange membranes, can solve the problems of high conductivity, low electrical conductivity, poor mechanical stability and chemical stability of anion exchange membranes, and achieves the Low production cost, high mechanical strength, and excellent film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
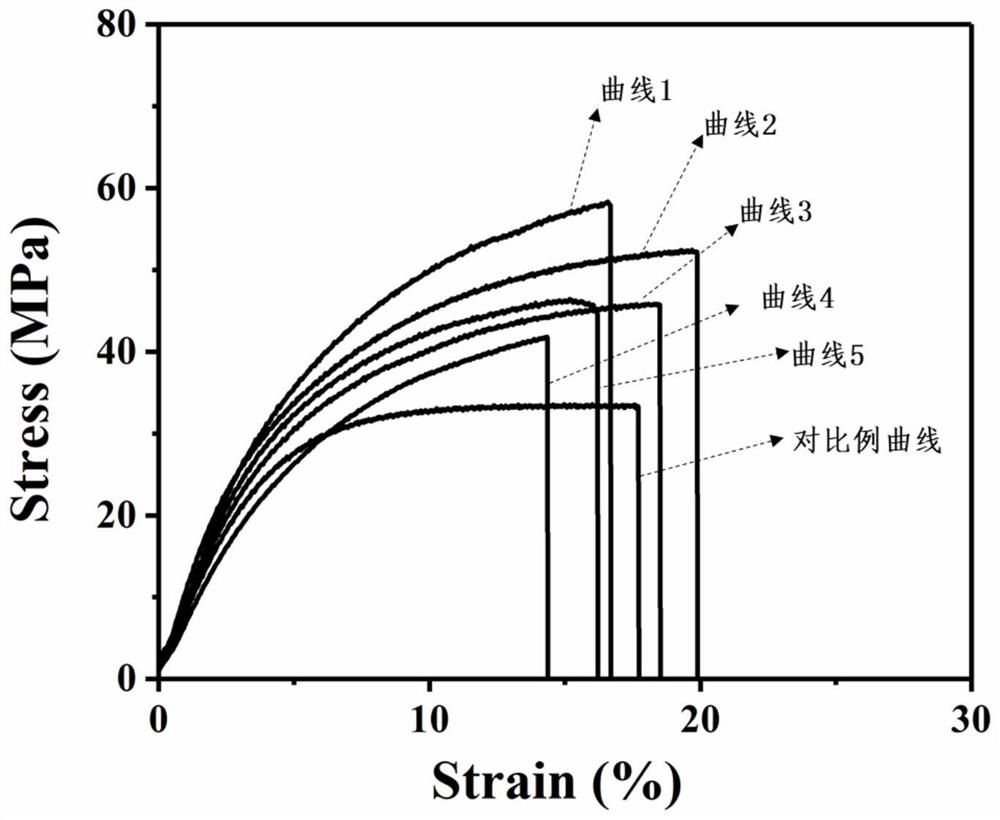
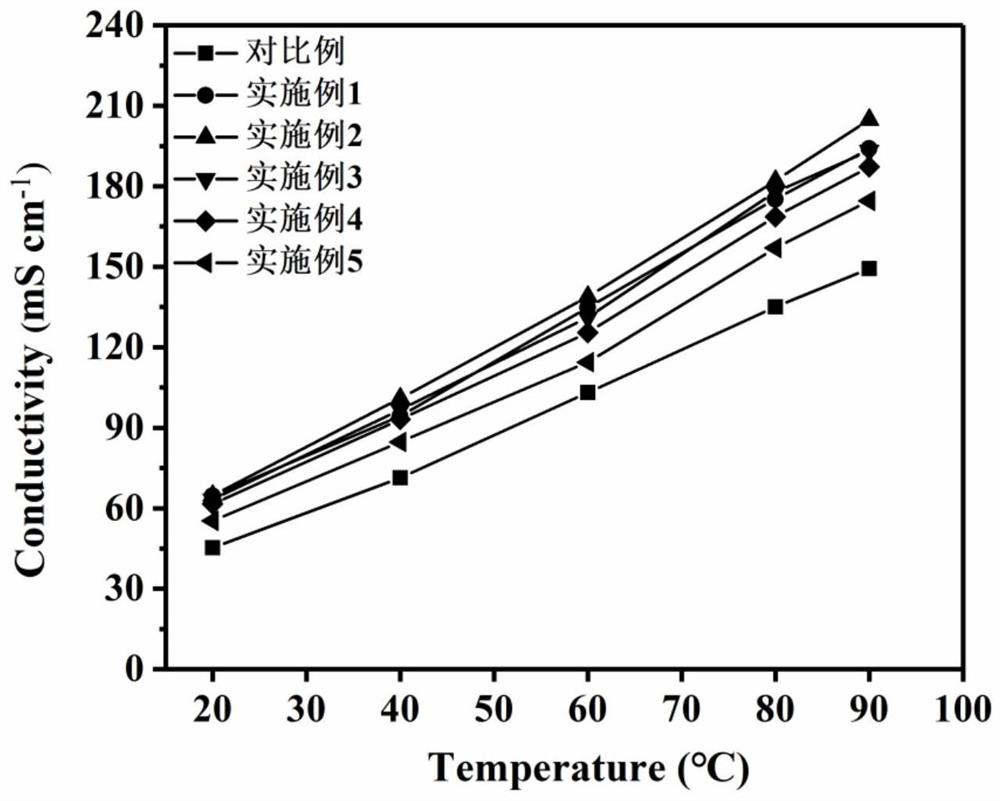
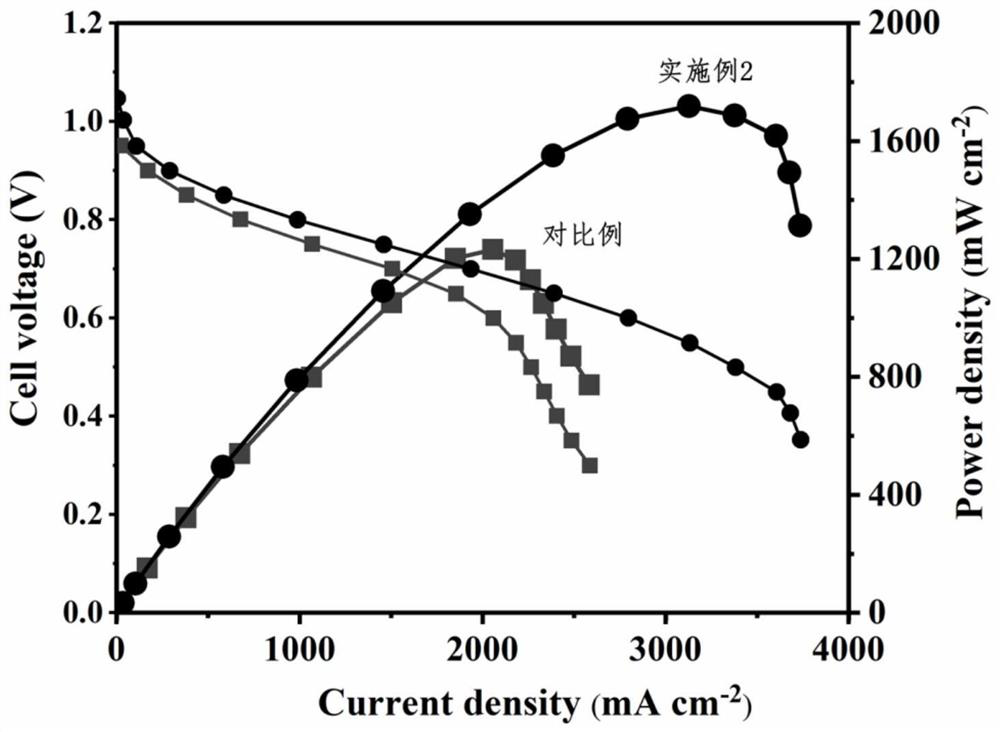
Examples
Embodiment 1
[0030] A kind of preparation method of carbazole polyaromatic piperidine anion exchange membrane, its concrete method step comprises:
[0031] (1), under normal temperature conditions, first dissolve N-ethylcarbazole and p-terphenyl monomer in chloroform according to the molar ratio of 5:95 to form a homogeneous or heterogeneous solution; then, add dichloro Add N-methyl-4-piperidone monomer to the methane solution, stir to dissolve, the molar ratio of the sum of N-ethylcarbazole and p-terphenyl monomer to N-methyl-4-piperidone monomer 1:1, the concentration of all monomers in the solution is 10wt%;
[0032] (2), under the environment of -4 ℃, add trifluoroacetic acid and trifluoromethanesulfonic acid successively to the solution of step (1), wherein the volume of trifluoroacetic acid, trifluoromethanesulfonic acid and chloroform in the solution The ratio is 1:5:10, and then react at this temperature for 8 hours; after the reaction, pour the solution into 1M KOH solution to ob...
Embodiment 2
[0038] A kind of preparation method of carbazole polyaromatic piperidine anion exchange membrane, its concrete method step comprises:
[0039] (1), under normal temperature conditions, first N-ethylcarbazole and p-terphenyl monomer are dissolved in dichloromethane according to the molar ratio of 10:90 to form a homogeneous or heterogeneous solution; then, to Add N-methyl-4-piperidone monomer in the dichloromethane solution, stirring and dissolving, the sum of N-ethylcarbazole and p-terphenyl monomer and N-methyl-4-piperidone monomer The molar ratio is 1:1.05, and the concentration of all monomers in the solution is 20wt%;
[0040] (2), under the environment of 0 ℃, in the solution of step (1), add dropwise trifluoroacetic acid and trifluoromethanesulfonic acid successively, wherein trifluoroacetic acid, trifluoromethanesulfonic acid and dichloromethane in the solution The volume ratio is 1:8:10, and then react at this temperature for 12 hours; after the reaction, pour the sol...
Embodiment 3
[0048] A kind of preparation method of carbazole polyaromatic piperidine anion exchange membrane, its concrete method step comprises:
[0049] (1), under normal temperature conditions, first dissolve N-ethylcarbazole and p-terphenyl monomer in chloroform according to the molar ratio of 15:85 to form a homogeneous or heterogeneous solution; then, add dichloro Add N-methyl-4-piperidone monomer to the methane solution, stir to dissolve, the molar ratio of the sum of N-ethylcarbazole and p-terphenyl monomer to N-methyl-4-piperidone monomer 1:1.1, the concentration of all monomers in the solution is 25wt%;
[0050] (2), under the environment of 3 ℃, in the solution of step (1), add dropwise trifluoroacetic acid and trifluoromethanesulfonic acid successively, wherein the volume ratio of trifluoroacetic acid, trifluoromethanesulfonic acid and chloroform in the solution 1:8:8, then react at this temperature for 24h; after the reaction, pour the solution into 1M KOH solution to obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mechanical strength | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com