Preparation method of high-purity sennoside and sennoside or derivative prepared by same
A kind of sennoside, high-purity technology, applied in the field of preparation of sennoside or derivatives, high-purity sennoside, can solve the problems of easy decomposition, troublesome decoction decoction, complicated operation procedures, etc., and achieves easy scale-up production , The cost of the solution is high, and the separation effect is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
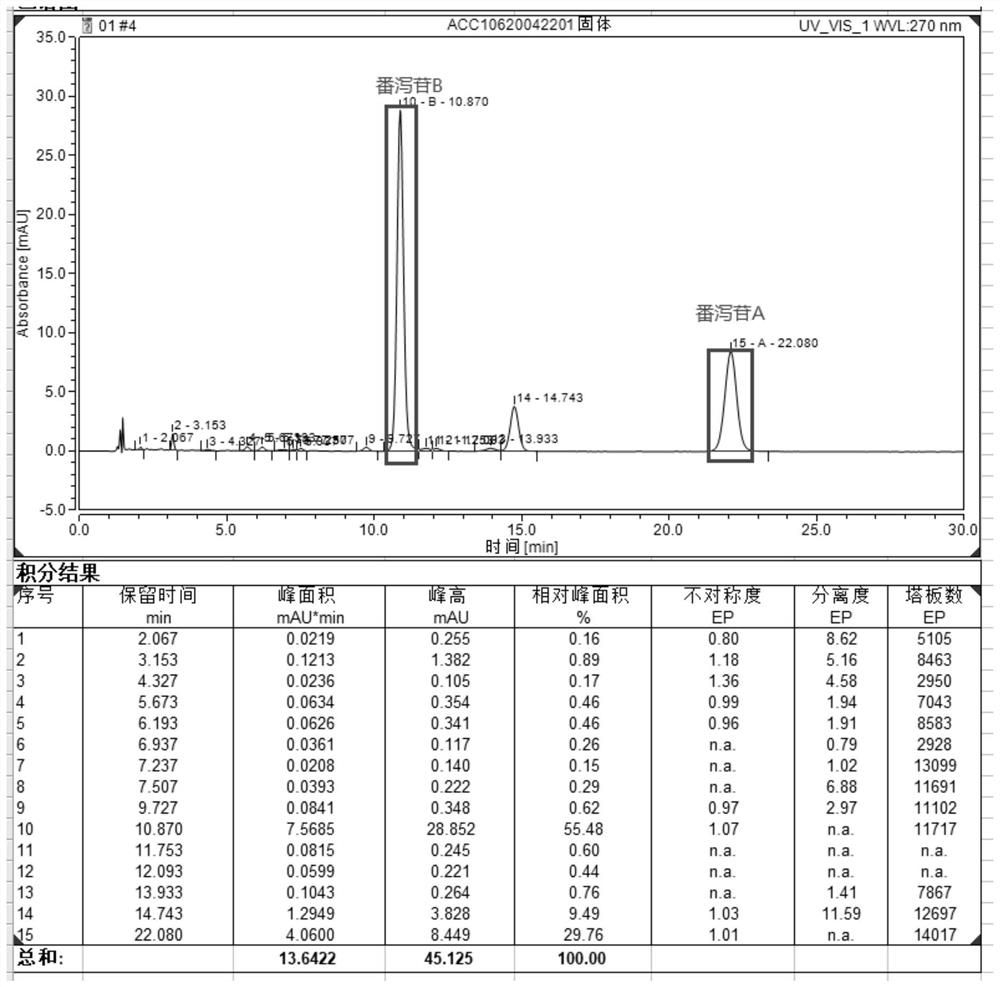
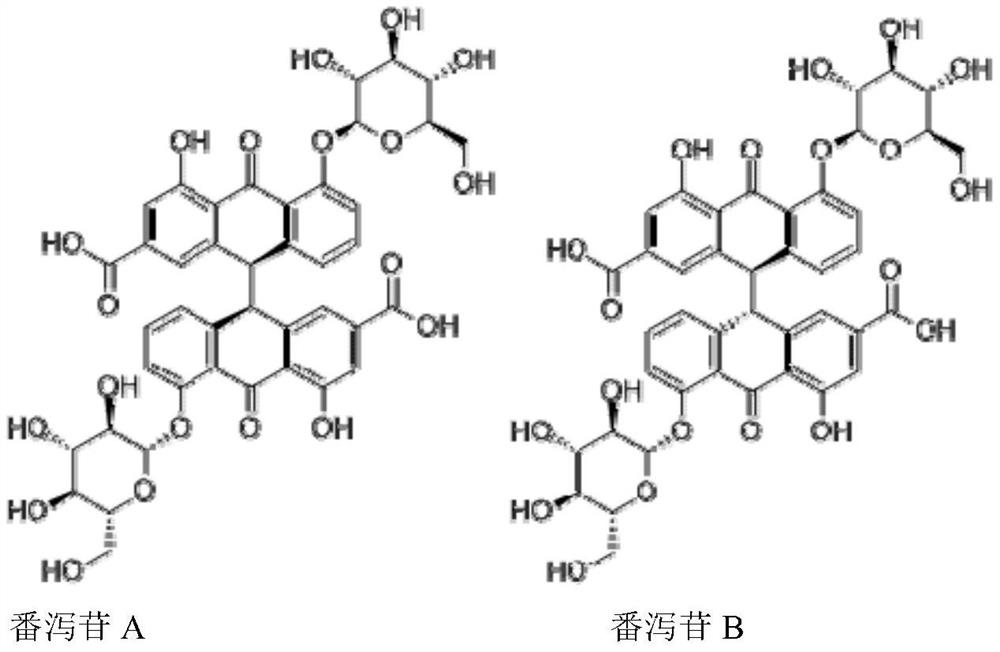
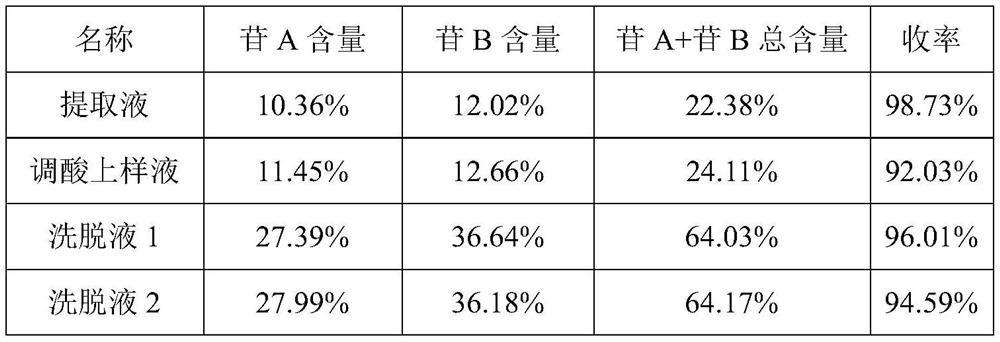
[0046] Embodiment 1: (1) Medicinal material extraction: get 100g senna medicinal material (wherein sennoside A+sennoside B content is 1.25%) add 8 times (g / mL) 0.1% sodium bicarbonate solution stirring extraction 60min after filtering , leave the supernatant as the extract (extract sampling test), add 6mol / L HCl to adjust the pH to 3.0, remove some impurities, filter and leave the supernatant, add sodium chloride to adjust the conductivity to 20mS / cm, add 5mol / L L NaOH adjusts pH to be 3.5, obtains sample solution (acid adjustment sample solution sampling detection);
[0047] (2) Ion-exchange column chromatography: 300 mL of A451 (ZG A451 type, Hangzhou Zhengguang Resin Co., Ltd.) macroporous weakly basic anion-exchange chromatography column is pre-installed, and the column is equilibrated 5 times with equilibrium solution A at a linear velocity of 60 cm / h Volume, balance liquid A is 0.02mol / L glycine buffer solution, contains 0.2mol / L NaCl, pH is 3.5, and conductivity is 23mS...
Embodiment 2
[0054] Embodiment 2: (1) medicinal material extraction: get 100g senna medicinal material (wherein sennoside A+sennoside B content is 1.23%) add 10 times (g / mL) 0.25% sodium bicarbonate solution and stir to extract 90min after filtering , leave the supernatant as the extract (extract sample detection), add glacial acetic acid to adjust the pH to 4.5, filter and leave the supernatant, add sodium chloride to adjust the conductance to 15mS / cm, add 5mol / L NaOH to adjust the pH to 7.0, Obtain the sample solution (acid adjustment sample solution sampling detection);
[0055] (2) Ion-exchange column chromatography: 300mL macroporous weakly basic anion-exchange chromatography column of A351 type (ZG A351 type, Hangzhou Zhengguang Resin Co., Ltd.) is pre-installed, and the column is equilibrated 5 times with equilibrium solution A at a linear velocity of 60cm / h Volume, balance solution A is 0.02mol / L phosphate buffer, containing 0.15mol / L NaCl, pH is 7.0, conductivity is 18mS / cm, then ...
Embodiment 3
[0063] Embodiment 3: (1) medicinal material extraction: get 1000g senna medicinal material (wherein sennoside A+sennoside B content is 1.15%), add 10 times (g / mL) 0.25% sodium bicarbonate solution and stir to extract 120min after filtration , leave the supernatant as the extract (extract sample detection), add 6mol / L HCl to adjust the pH to 5.5, filter and leave the supernatant, add sodium chloride to adjust the conductivity to 7mS / cm, add 5mol / L NaOH to adjust the pH to 10.0, to obtain the sample solution (acid adjustment sample solution sampling detection);
[0064] (2) Ion-exchange column chromatography: 1500mL macroporous weakly basic anion-exchange chromatography column of A313 type (ZG A313 type, Hangzhou Zhengguang Resin Co., Ltd.) is pre-installed, and the column is equilibrated 5 times with equilibrium solution A at a linear velocity of 60cm / h Volume, balance liquid A is 0.2mol / L sodium bicarbonate buffer solution, contains 0.1mol / L NaCl, pH is 10.0, and conductivity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com