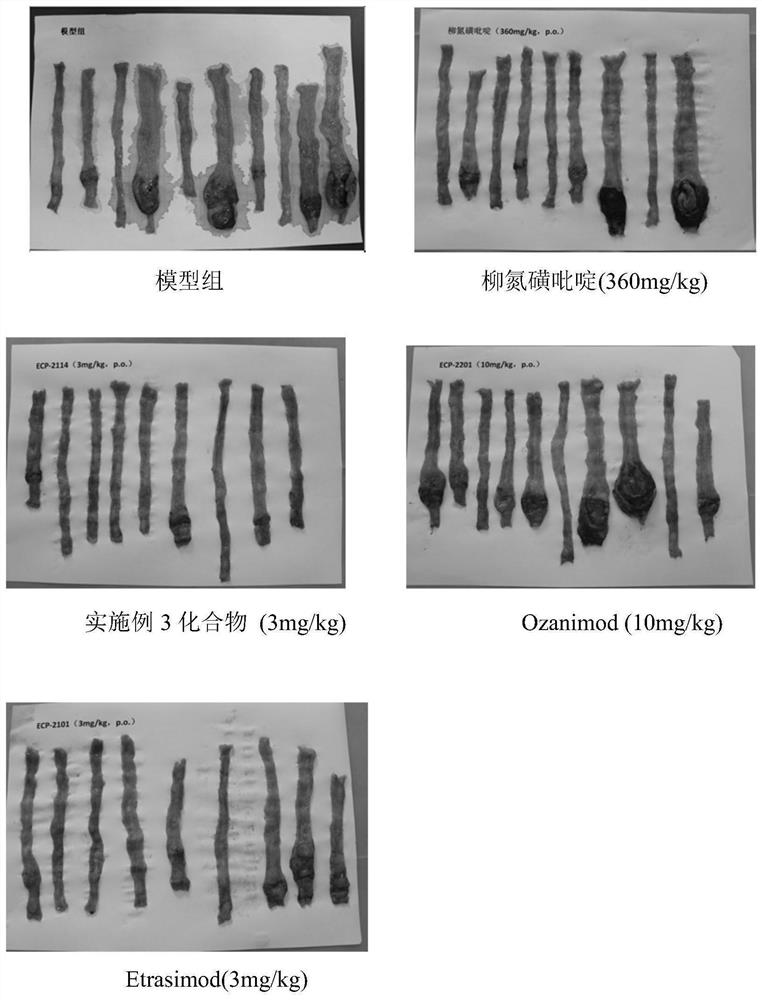
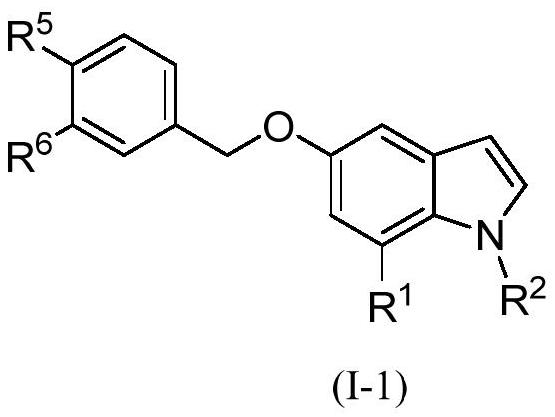
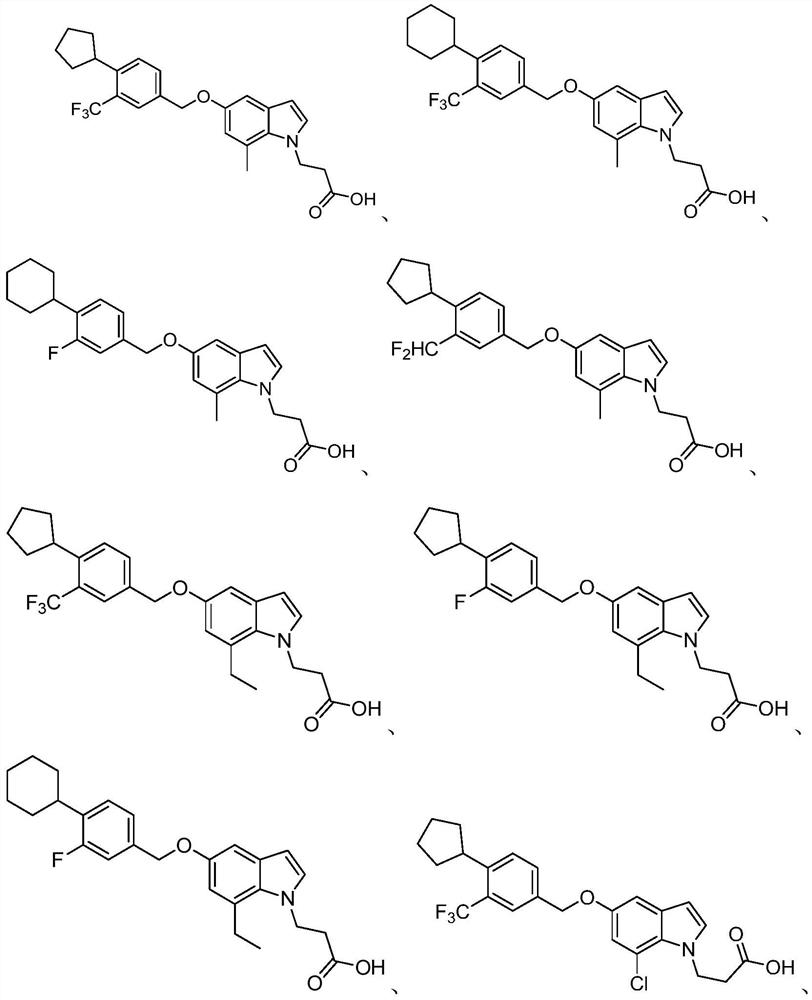
Indole derivative and application thereof
A technology of indole derivatives and compounds, applied in the field of medicinal chemistry, can solve problems such as unmet medical needs, and achieve the effect of novel structure, good drug effect and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] 3-(5-((4-cyclopentyl-3-(trifluoromethyl)benzyl)oxy)-7-methyl-1H-indol-1-yl)propanoic acid (1)
[0115]
[0116] The first step: 1-(2-amino-5-methoxy-3-methylphenyl)-2-chloroethanone (1b)
[0117]
[0118] Dissolve 2-methyl-4-methoxyaniline (1a) (5.0g, 36.4mmol) in toluene (20mL), and add boron trichloride (37mL, 1M dichloromethane solution) and Titanium tetrachloride (37 mL, 1M in dichloromethane) and chloroacetonitrile (3.1 g, 41 mmol). The reaction solution was heated to 90°C and stirred for 3h. A 2M aqueous hydrochloric acid solution (30 mL) was added to the reaction system, and stirred at 85° C. for 45 min. Saturated sodium bicarbonate solution (100 mL) was added to the reaction system, and extracted with dichloromethane (100 mL×3). The organic phase was dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (ethyl acetate:petroleum ether (v / v)=1:5...
Embodiment 2
[0143] 3-(5-((4-cyclopentyl-3-(trifluoromethyl)benzyl)oxy)-7-ethyl-1H-indol-1-yl)propanoic acid (2)
[0144]
[0145]
[0146] The first step: 2-ethyl-4-methoxy-1-nitrobenzene (2b)
[0147]
[0148] To a solution of 1-methoxy-4-nitrobenzene 2a (5 g, 32.65 mmol) in tetrahydrofuran (100 mL) was added 1M vinylmagnesium bromide solution (75.10 mL) at -15°C. The reaction solution was stirred at -15 °C for 2 h. 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (12.60 g, 55.51 mmol) was added, and the reaction solution was stirred at -40° C. for 3 h. Saturated ammonium chloride aqueous solution (50 mL) was added to the reaction liquid, and extracted with ethyl acetate (100 mL×3). The organic phase was dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (ethyl acetate:petroleum ether (v / v)=1:5) to obtain a dark yellow oil 2-ethyl- 4-Methoxy-1-nitrobenzene 2b (2.55 g, y...
Embodiment 3
[0172] 3-(7-Chloro-5-((4-cyclopentyl-3-(trifluoromethyl)benzyl)oxy)-1H-indol-1-yl)propanoic acid (3)
[0173]
[0174] The first step: 2-chloro-4-((4-cyclopentyl-3-(trifluoromethyl)benzyl)oxy)-1-nitrobenzene (3b)
[0175]
[0176] Add 4-(chloromethyl)-1-cyclopentyl-2- (Trifluoromethyl)benzene (1.51 g, 5.76 mmol) and cesium carbonate (3.75 g, 11.52 mmol). Reaction at 80°C for 3h. The reaction solution was diluted with water (20 mL), extracted with ethyl acetate (30 mL×3). The organic phase was dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (ethyl acetate:petroleum ether (v / v)=1:20) to obtain 2-chloro-4- ((4-cyclopentyl-3-(trifluoromethyl)benzyl)oxy)-1-nitrobenzene (3b) (2.1 g, yield: 91.2%).
[0177] MS (ESI) m / z: 400 [M+1].
[0178] The second step: 7-chloro-5-((4-cyclopentyl-3-(trifluoromethyl)benzyl)oxy)-1H-indole (3c)
[0179]
[0180]Add 2-c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com