Synthesis method of bremelanotide acetate
A technology of bremelanotide acetate and its synthesis method, which is applied in the field of polypeptide production, can solve problems such as large steric hindrance of peptide chains, difficulty in forming rings, and restrictions on the application of bremelanotide, so as to improve the purification yield and purity, and achieve chromatographic Good symmetry of peak shape and improved separation selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] A preparation method of RinkAmide resin modified by 4-trifluoromethylmandelic acid:
[0077] 1) Rinse 100 g of RinkAmide resin (with a substitution degree of 0.45 mmol / g and a cross-linking degree of 1%) with DCM for 5 times, soak in 500 mL of DCM for 20 min to fully swell, and add the resin to 500 mL of piperidine / DCM mixed solution (the mass percentage of piperidine is 25%), stirred and reacted for 30min to de-Fmoc protect the resin;
[0078] 2) Add 22 g of 4-trifluoromethylmandelic acid and 25.4 g of HOBt into 300 mLDCM and stir to dissolve, pre-cool at 0°C for 10 min, add 15.2 g of DIC and stir for 10 min, then add resin, stir at room temperature for 2.5 h, filter , washed with DCM 6 times, that is.
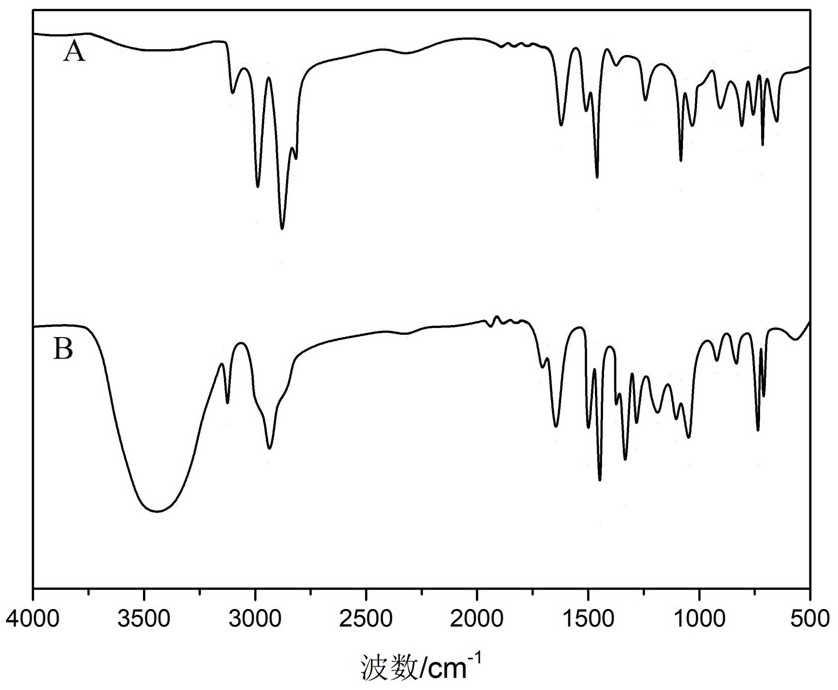
[0079] The RinkAmide resin modified by RinkAmide resin and 4-trifluoromethylmandelic acid is carried out to infrared spectroscopic analysis, and the measured results are as follows: figure 1 shown.
[0080] observe figure 1 , A represents the unmodified RinkAmide ...
Embodiment 2
[0082] A preparation method of bremelanotide resin, said method comprising:
[0083] 1) Preparation of Fmoc-Lys(Boc)-resin:
[0084] Using the RinkAmide resin modified with 4-trifluoromethylmandelic acid obtained in Example 1 as a carrier, the resin was added to the piperidine / DCM mixed solution (the mass percentage of piperidine was 25%) and reacted for 20 min, rinsed with DCM 5 times; mix 70.2g Fmoc-Lys(Boc)-OH with 36g HOBt, 300 mLDCM, stir to dissolve, pre-cool at 0°C for 10 minutes, add 21.5g DIC and stir for 8 minutes, then add to the resin, stir and react for 10 minutes , add 2 gDMAP, stir and react at room temperature for 3.5h, add 46 g acetic anhydride, 35.5 g pyridine, react at room temperature for 40 min, wash 6 times with DCM, and obtain Fmoc-Lys(Boc)-resin shown in formula (1) ,
[0085] (1);
[0086] 2) Preparation of Fmoc-Trp(Boc)-Lys(Boc)-resin:
[0087] The Fmoc-Lys(Boc)-resin obtained in step 1) was added to the piperidine / DCM mixed solution (the mass ...
Embodiment 3
[0110] A kind of preparation method of bremelanotide crude product:
[0111] 1) Cracking: Mix TFA:TIS:deionized water according to the mass ratio of 93:3:4 to obtain a cracking agent, pre-cool at -20°C for 1 h, add the bremelanotide resin obtained in Example 2 to 1 L cracking agent solution, reacted at 0°C for 1 h, raised to room temperature and reacted for 3 h to remove the resin, filtered off the resin, and added the filtrate to 14 L pre-frozen methyl tert-butyl ether under stirring conditions, a large amount of white matter was precipitated, centrifuged, and collected The resulting solid was centrifuged, washed with methyl tert-butyl ether until neutral, and dried;
[0112] 2) Ester bond hydrolysis: Step 1) Dissolve the product in 20wt% acetonitrile aqueous solution, adjust pH=12 with 1 mol / L NaOH aqueous solution under ice-bath stirring condition, react at 0°C for 0.5 h, raise to 30°C to continue reaction 1 h, centrifuge to remove the precipitated white substance, adjust ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com