Method for continuously synthesizing chlorobutane in non-aqueous system
A technology of chlorobutane and system, which is applied in the field of continuous synthesis of chlorobutane, can solve the problems of low efficiency and high energy consumption, and achieve the effects of shortening the process flow, simple operation and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the method for the continuous synthesis of chlorobutane in a kind of non-aqueous system
[0019] Include the following steps:
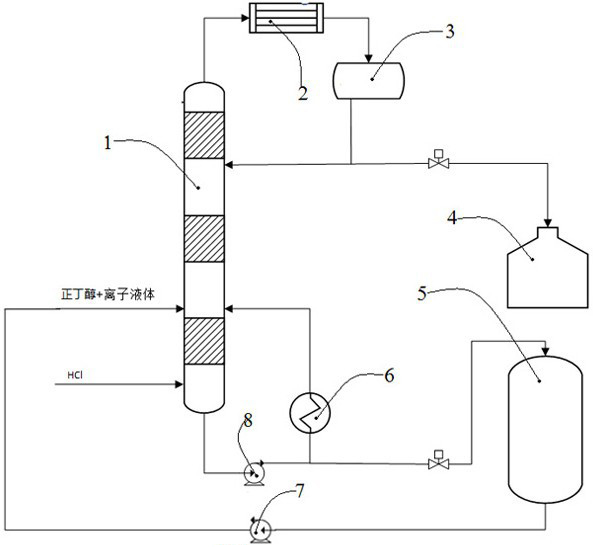
[0020] Add 10 kilograms of 1-butyl-3-methylimidazolium chloride salt at the bottom of the reactive distillation column 1 and pass it into the reboiler 6 for circulation, open the reboiler 6 to make the whole system of the reactive distillation column 1 warm up to 120 ± 2 ℃, and then pass 1-butyl-3-methylimidazolium chloride into the falling film evaporator 5 to raise the temperature to 110 ℃. Open the second circulating pump 7, and the ionic liquid enters the reactive distillation tower 1 in the form of spray from the top of the first tower section of the reactive distillation tower 1 after the falling film evaporator 5, and the flow rate of the spray is 210g / min, and then At the top of the first tower section, n-butanol was introduced at a flow rate of 74g / min, and hydrogen chloride gas was introduced at the bottom of the tower ...
Embodiment 2
[0023] Add 10 kg of 1-butyl-3-methylimidazolium chloride salt to the bottom of reactive distillation tower 1, the temperature in the entire system of reactive distillation tower 1 is raised to 90±2°C, the temperature of falling film evaporator 5 is raised to 100°C, other processes The conditions were the same as in Example 1, and reflux began to occur at the top of the tower after 30 minutes of feeding hydrogen chloride gas.
[0024] Example 2, take an instant sample (initial distillation) at the top of the tower after 30 minutes of feeding for analysis, the content of 1-chlorobutane is 95.56%, the content of n-butanol is 2.87%, n-butyl ether is 1.110%, and isomers are 0.082%. Others 0.378%; 1 hour and 45 minutes after the introduction, take an instant sample from the top of the tower for analysis, 1-chlorobutane content 87.13%, n-butanol content 11.18%, n-butyl ether 0.48%, isomers 0.047%, other 1.163 %; 10 hours after the introduction, take an instant sample from the top of ...
Embodiment 3
[0026] Add 10 kg of 1-butyl-3-methylimidazolium chloride salt to the bottom of reactive distillation tower 1, the temperature in the entire system of reactive distillation tower 1 is raised to 150±2°C, the temperature of falling film evaporator 5 is raised to 120°C, and the top of the tower The reflux temperature was controlled at 90±1° C., and the other process conditions were the same as in Example 1. The reflux began to appear at the top of the tower after 30 minutes of hydrogen chloride gas feeding.
[0027] Example 3, take an instant sample (initial distillation) at the top of the tower after 30 minutes for analysis, the content of 1-chlorobutane is 99.87%, the content of n-butanol is 0.049%, n-butyl ether is 0.0094%, and isomers are 0.0512%. Others 0.0204%; 1 hour and 45 minutes after the introduction, take an instant sample from the top of the tower for analysis, 1-chlorobutane content 98.74%, n-butanol content 0.23%, n-butyl ether 0.0113%, isomers 0.141%, other 0.8777 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com