Water-stable microporous difunctional MOFs material as well as preparation method and application thereof
A water-stable, dual-function technology, applied in analytical materials, material excitation analysis, chemical instruments and methods, etc., can solve problems such as difficult solvent degradation, secondary environmental pollution, poor stability, etc., to enhance adsorption performance, fluorescence performance. The effect of good, good water stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Accurately weigh H 6 OBTEC 13.5mg (0.05mmol), then add to a 10mL glass bottle (sample bottle); then add 5mL deionized water and 1mol / L diethylamine solution 175uL (0.175mmol), sonicate until the organic ligand is completely dissolved , then add 1mol / L of Zn(NO 3 ) 2 ·6H 2 O 0.15mmol, sonicate again to mix evenly. Finally, put the sample bottle into a steel kettle lined with 25mL polytetrafluoroethylene, keep the steel kettle in an oven at 120°C for 96 hours, cool down to room temperature for 24 hours, wash the product three times with deionized water, and air-dry , obtain colorless transparent crystal, productive rate is about 65% (with H 6 OBTEC calculation).
Embodiment 2
[0034] Embodiment 2: The reaction temperature is changed from 120° C. in Embodiment 1 to 110° C.
[0035] Accurately weigh H 6 OBTEC 13.5mg (0.05mmol), then add to a 10mL glass bottle (sample bottle); then add 5mL deionized water and 1mol / L diethylamine solution 175uL (0.175mmol), sonicate until the organic ligand is completely dissolved , then add 1mol / L of Zn(NO 3 ) 2 ·6H 2 O 0.15mmol, sonicate again to mix evenly. Finally, put the sample bottle into a steel kettle lined with 25mL polytetrafluoroethylene, keep the steel kettle in an oven at 110°C for 96 hours, cool down to room temperature for 24 hours, wash the product three times with deionized water, and air-dry , to obtain colorless transparent crystals, but compared to Example 1, the yield of Example 2 is about 60%.
Embodiment 3
[0036] Embodiment 3: The reaction temperature is changed from 120° C. in Embodiment 1 to 100° C.
[0037] Accurately weigh H 6 OBTEC 13.5mg (0.05mmol), then add to a 10mL glass bottle (sample bottle); then add 5mL deionized water and 1mol / L diethylamine solution 175uL (0.175mmol), sonicate until the organic ligand is completely dissolved , then add 1mol / L of Zn(NO 3 ) 2 ·6H 2 O 0.15mmol, sonicate again to mix evenly. Finally, put the sample bottle into a steel kettle lined with 25mL polytetrafluoroethylene, keep the steel kettle in an oven at 100°C for 96 hours, cool down to room temperature for 24 hours, wash the product three times with deionized water, and air-dry , Obtain a small amount of colorless transparent crystals, compared with Example 2, there are less white flocculent impurities in Example 3.
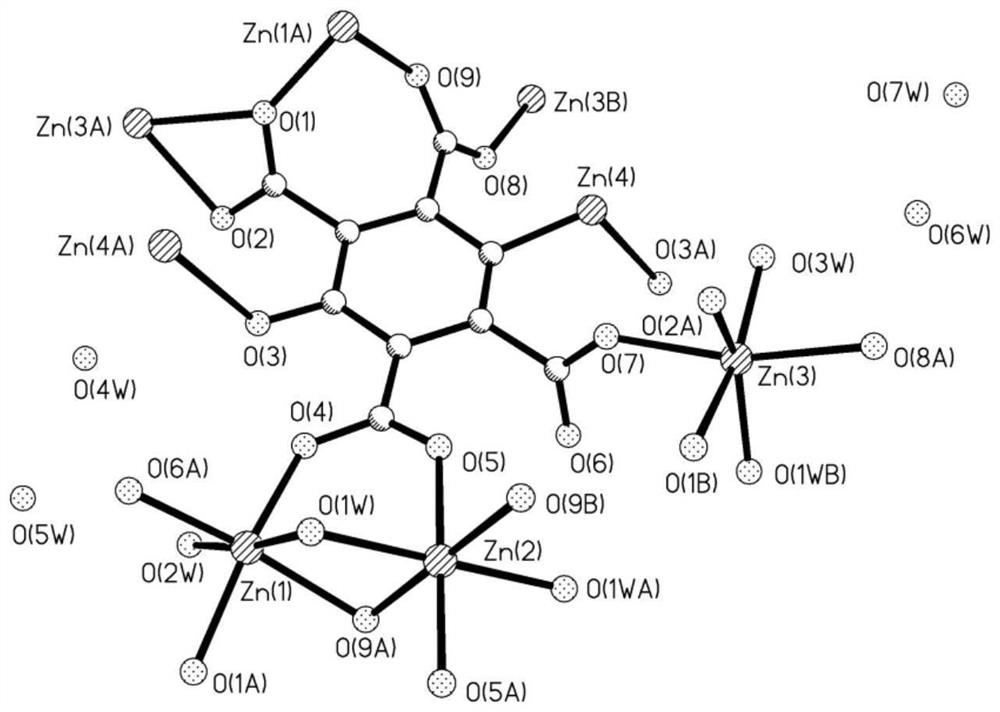
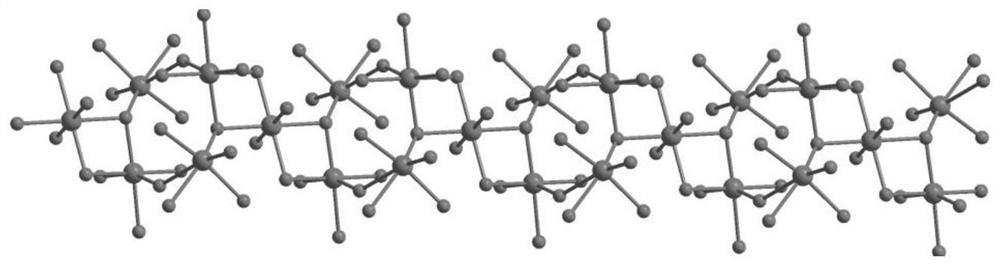
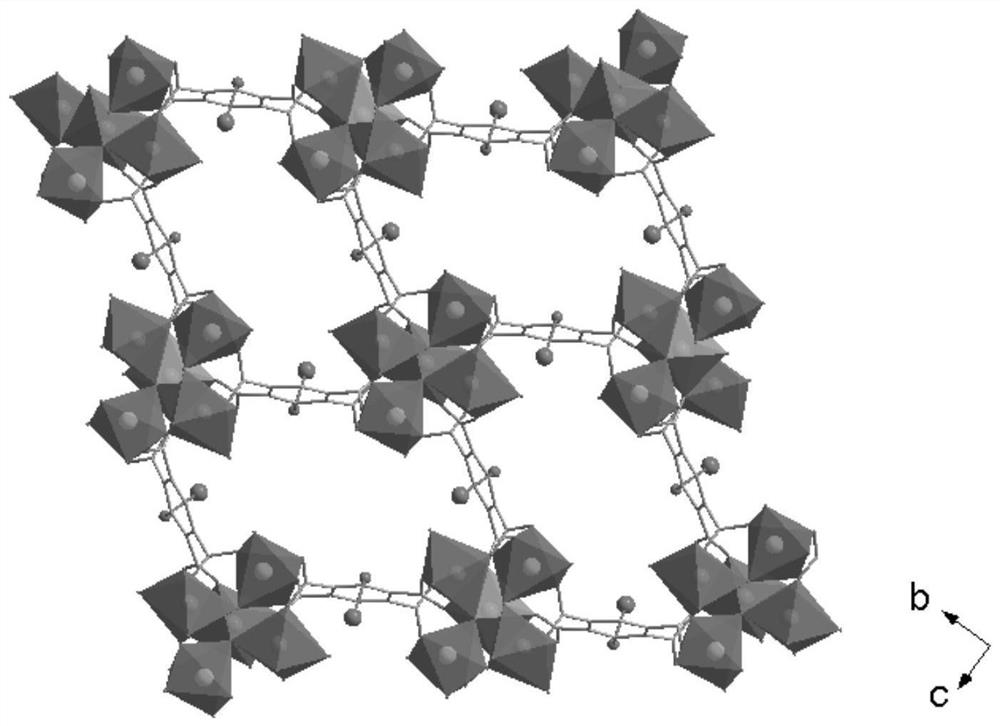
[0038] figure 1 It is the coordination environment diagram of the sample prepared in the present invention. There are four crystallographically independent Zn(II), a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com