Method for removing impurities in recycled acetic acid
A technology for acetic acid and impurities, applied in the fields of chemistry and chemical engineering, which can solve the problems of increased energy consumption of the production system, high maintenance frequency of reaction equipment and systems, increased production costs and maintenance costs, etc. The effect of decreasing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
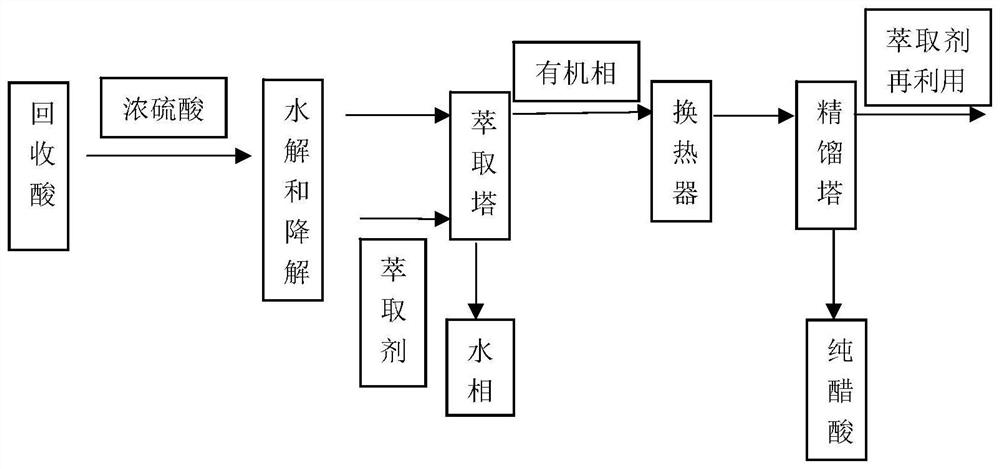
[0042] Take 100kg of acid recovered from cellulose acetate production (concentration is about 30%, about 70% is water), add 1kg of concentrated sulfuric acid (above 98%), and stir the system at 50 degrees for 4h, then add 1.45kg of magnesium acetate to neutralize sulfuric acid to form Magnesium acetate, the reaction was completed after about 0.2h. After the reaction, the degree of substitution of soluble cellulose diacetate, cellulose triacetate, unreacted complete cellulose DS and degree of polymerization DP decreased significantly in acetic acid, and there were soluble cellulose diacetate, cellulose triacetate, unreacted Completely reacted cellulose, the total amount is less than 1%, the main particle size distribution is below 35 μm, about 40% of the particles are below 5 μm, and the haze value of the solution is about 8.7. After the reaction, the hydrophilicity of the impurities is better, and more impurities are dissolved in acetic acid and distributed in water. After th...
Embodiment 2
[0045] Take 100kg of acid recovered from cellulose acetate production (concentration is about 30%, about 70% is water), add 2kg of concentrated sulfuric acid (above 98%), stir the system at 35 degrees for 4h, then add 2.9kg of magnesium acetate to neutralize sulfuric acid to form Magnesium acetate, the reaction was completed after about 0.2h. After the reaction, the degree of substitution of soluble cellulose diacetate, cellulose triacetate, unreacted complete cellulose DS and degree of polymerization DP decreased significantly in acetic acid, and there were soluble cellulose diacetate, cellulose triacetate, unreacted Completely reacted cellulose, the total amount is less than 1%, the main particle size distribution is below 25 μm, about 40% of the particles are below 5 μm, and the haze value of the solution is about 7.6. After the reaction, the hydrophilicity of the impurities is better, and more impurities are dissolved in acetic acid and distributed in water. After the rea...
Embodiment 3
[0048] Take 100kg of recovered acid from cellulose acetate production (the concentration of acetic acid is about 30%, and about 70% is water), add 2kg of concentrated sulfuric acid (above 98%), stir the system at 50 degrees for 4h, and then add 2.9kg of magnesium acetate to neutralize the sulfuric acid Magnesium acetate was formed and the reaction was complete after about 0.2 h. After the reaction, the degree of substitution of soluble cellulose diacetate, cellulose triacetate, unreacted complete cellulose DS and degree of polymerization DP decreased significantly in acetic acid, and there were soluble cellulose diacetate, cellulose triacetate, unreacted Completely reacted cellulose, the total amount is less than 1%, the main particle size distribution is below 20 μm, about 50% of the particles are below 5 μm, and the haze value of the solution is about 1.7. After the reaction, the hydrophilicity of the impurities is better, and more impurities are dissolved in acetic acid and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com