Docetaxel for injection and preparation method thereof
A technology for docetaxel and injection, which is applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc., can solve the problems of low stability of nano-microspheres and improve the encapsulation efficiency and drug loading, optimizing pH regulation, and enhancing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
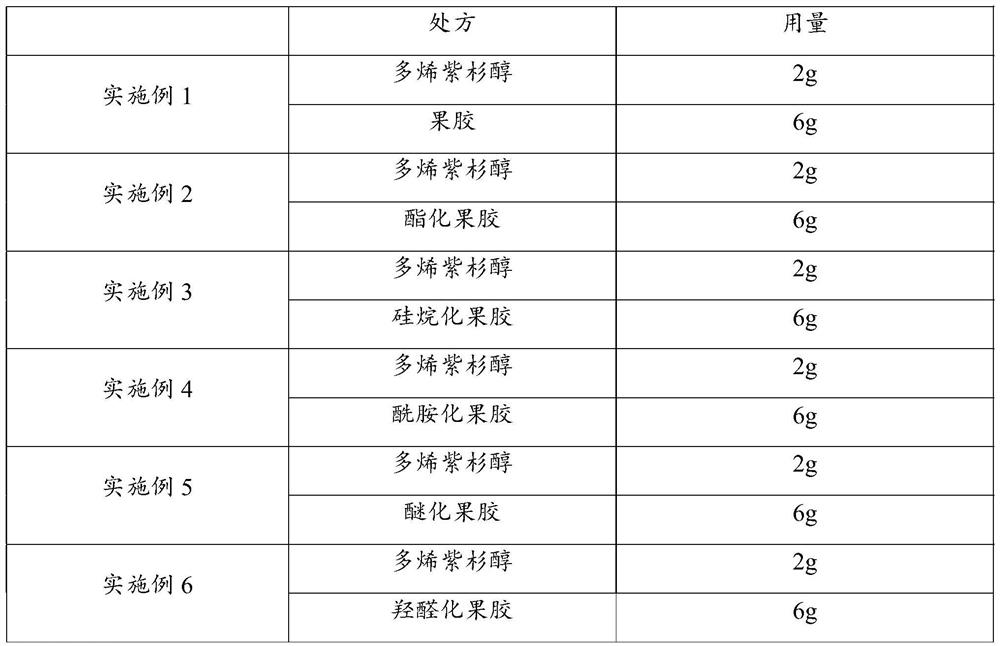
[0026] Embodiment 1-6 docetaxel nano microspheres
[0027]
[0028] Preparation:
[0029] (1) dissolving the carrier material in a glycine-citric acid buffer solution with a pH of 4.0 at 30-40° C., adding 20 ml of dimethyl sulfoxide to aid dissolution, and preparing a 1%-5% solution to obtain a solution A;
[0030] (2) Slowly add docetaxel to solution A while stirring, and stir and reflux in a water bath at 25-30°C for 1-1.5h to obtain solution B;
[0031] (3) The solution B is dialyzed for 4-8 hours with a dialysis bag, and the outer solution of the dialysis is replaced every 2 hours, and the obtained nano-microspheres in the bag are vacuum-dried to obtain the final product.
[0032] Embodiment 1-6 is the screening optimization process to drug-loaded material, and its encapsulation efficiency is all greater than 80%, and drug loading capacity is all greater than 35%, and the docetaxel nano-microsphere encapsulation efficiency and drug loading capacity of embodiment 2 are ...
Embodiment 7-14
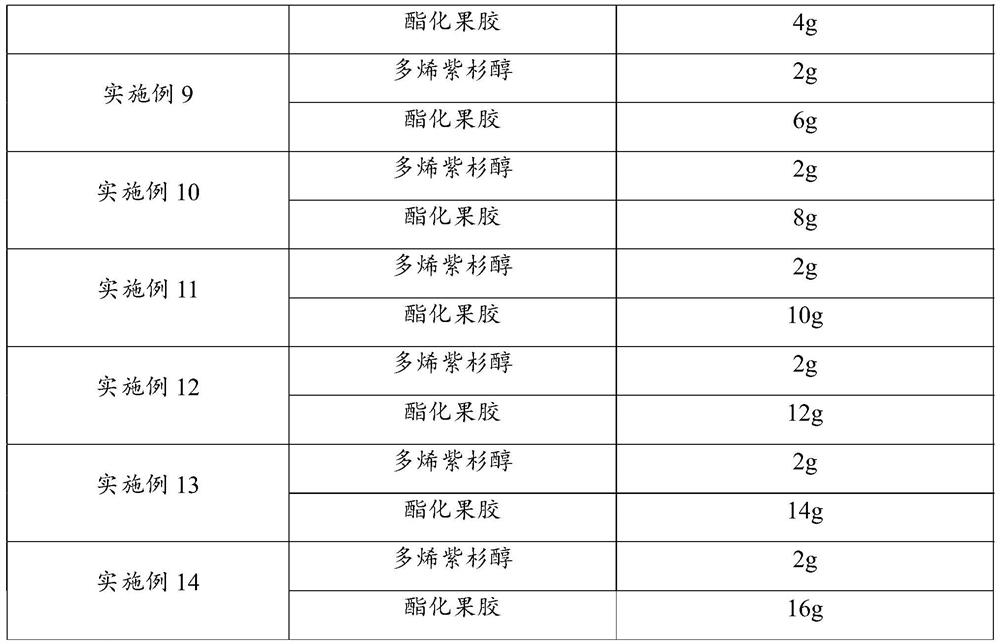
[0033] Embodiment 7-14 docetaxel nano microspheres
[0034]
[0035]
[0036] Preparation:
[0037] (1) dissolving the carrier material in a glycine-citric acid buffer solution with a pH of 4.0 at 30-40° C., adding 20 ml of dimethyl sulfoxide to aid dissolution, and preparing a 1%-5% solution to obtain a solution A;
[0038] (2) Slowly add docetaxel to solution A while stirring, and stir and reflux in a water bath at 25-30°C for 1-1.5h to obtain solution B;
[0039] (3) The solution B is dialyzed for 4-8 hours with a dialysis bag, and the outer solution of the dialysis is replaced every 2 hours, and the obtained nano-microspheres in the bag are vacuum-dried to obtain the final product.
[0040] Examples 7-14 are experimental screening of the dosage ratio of docetaxel and esterified pectin in docetaxel nano-microspheres, the encapsulation efficiency is greater than 80%, and finally found that the weight ratio of docetaxel and esterified pectin In the range of 1:2-5, the...
Embodiment 15-21
[0041] Embodiment 15-21 docetaxel nano microspheres
[0042]
[0043]
[0044] Preparation:
[0045] (1) dissolving the carrier material in a glycine-citric acid buffer solution with a pH of 4.0 at 30-40° C., adding 20 ml of dimethyl sulfoxide to aid dissolution, and preparing a 1%-5% solution to obtain a solution A;
[0046] (2) Slowly add docetaxel to solution A while stirring, and stir and reflux in a water bath at 25-30°C for 1-1.5h to obtain solution B;
[0047] (3) The solution B is dialyzed for 4-8 hours with a dialysis bag, and the outer solution of the dialysis is replaced every 2 hours, and the obtained nano-microspheres in the bag are vacuum-dried to obtain the final product.
[0048] Examples 15-21 are to screen the degree of esterification of the drug-loaded material esterified pectin. In the experiment, the esterified pectin with a degree of esterification of 60%-75% has a better shape during the preparation process and is less prone to aggregation and coa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com