Method for efficiently oxidizing and synchronously removing iron and cobalt from nickel electrolysis anolyte and preparing magnetic material
A technology of magnetic materials and anolyte, applied in chemical instruments and methods, nickel compounds, inorganic chemistry, etc., can solve the problems of low primary utilization rate of resources, affecting product quality, adverse downstream production processes, etc., to improve the utilization efficiency of oxidants, The effect of impurity purification is good, and the effect of separation of nickel and cobalt is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
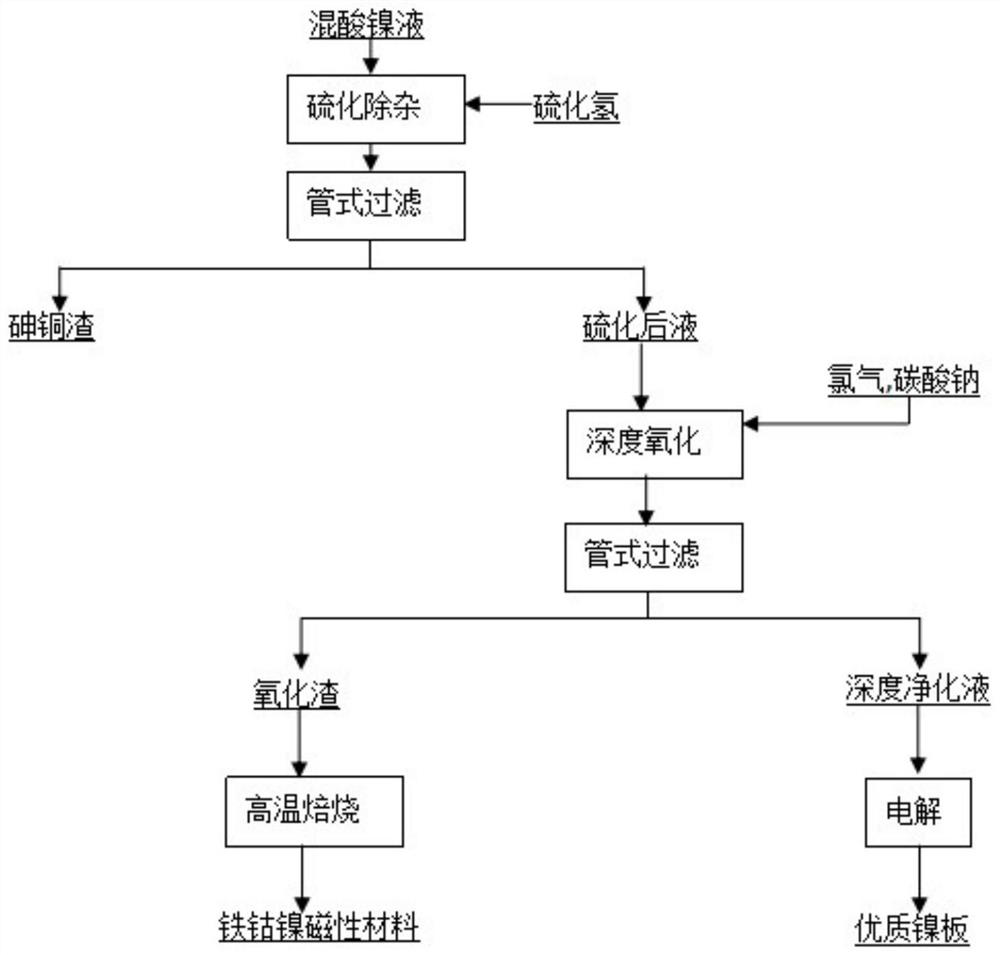
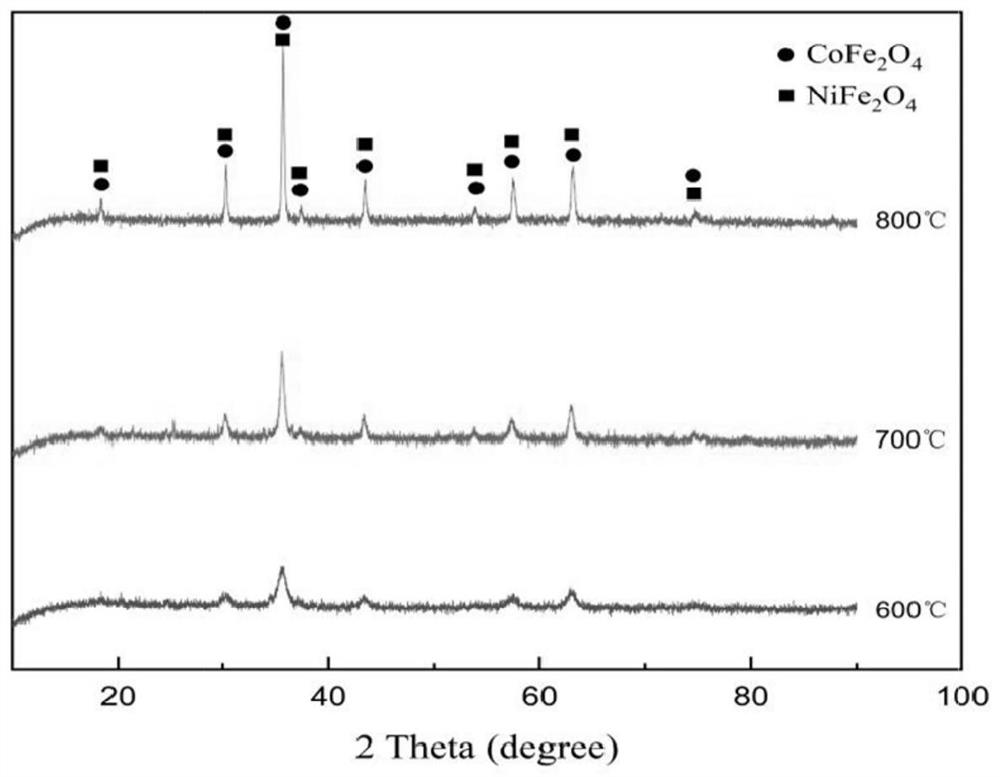
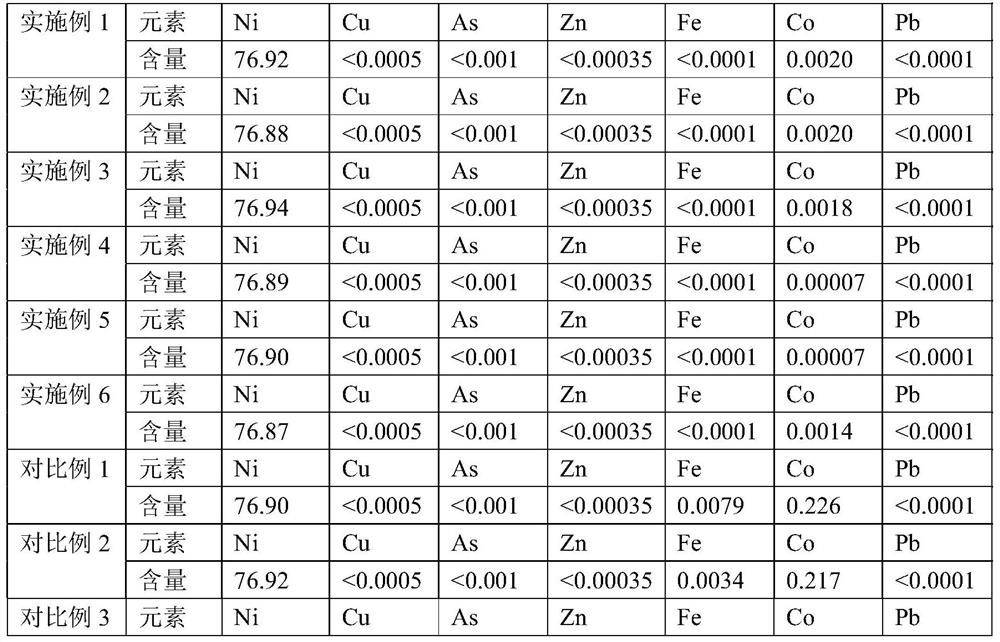
[0037] Pass into the mixed gas of chlorine and air in the nickel electrolysis anolyte to obtain the reaction solution, the amount of passing through the gas containing oxidizer per minute in every liter of mixed acid nickel solution is 0.35 liters, and the concentration of chlorine in the described mixed gas is 6.5%, adjust The pH of the reaction solution is 3.0, and the potential of the reaction solution is controlled to be 0.21V. The first stage of the reaction is carried out at 70°C. The time of the first stage of the reaction is controlled to be 44min, and the pH of the end point of the first stage of the reaction is 3.5. 1.1V, carry out the second-stage reaction at 70°C, control the time of the second-stage reaction to 92min, and control the pH at the end of the second-stage reaction to 4.0, and obtain the oxidized liquid and oxidation residue.
Embodiment 2
[0039] Pass into the mixed gas of chlorine and air in the nickel electrolysis anolyte to obtain the reaction solution, the amount of passing through the gas containing oxidizer per minute in every liter of mixed acid nickel solution is 0.35 liters, and the concentration of chlorine in the described mixed gas is 6.5%, adjust The pH of the reaction solution is 3.0, and the potential of the reaction solution is controlled to be 0.21V. The first stage of the reaction is carried out at 70°C, the time of the first stage of the reaction is controlled to be 50min, and the pH of the first stage of the reaction is 3.5, and then the potential of the reaction solution is controlled. 1.1V, the second stage reaction was carried out at 70°C, the time of the second stage reaction was controlled to be 82min, and the pH at the end of the second stage reaction was controlled to be 4.5 to obtain the oxidized liquid and oxidation residue.
Embodiment 3
[0041] Pass into the mixed gas of chlorine and air in the nickel electrolysis anolyte to obtain the reaction solution, the amount of passing through the gas containing oxidizer per minute in every liter of mixed acid nickel solution is 0.35 liters, and the concentration of chlorine in the described mixed gas is 6.5%, adjust The pH of the reaction solution is 3.0, and the potential of the reaction solution is controlled to be 0.21V. The first stage of the reaction is carried out at 70 ° C. The time of the first stage of the reaction is controlled to be 51 minutes. The pH of the end point of the first stage of the reaction is 3.5, and then the potential of the reaction solution is controlled. The temperature is 1.05V, and the second-stage reaction is carried out at 70°C. The time of the second-stage reaction is controlled to be 90 minutes, and the pH at the end of the second-stage reaction is controlled to be 5.0 to obtain the oxidized liquid and oxidation slag.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com