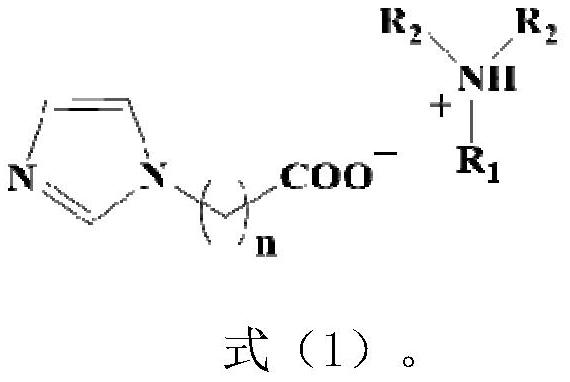
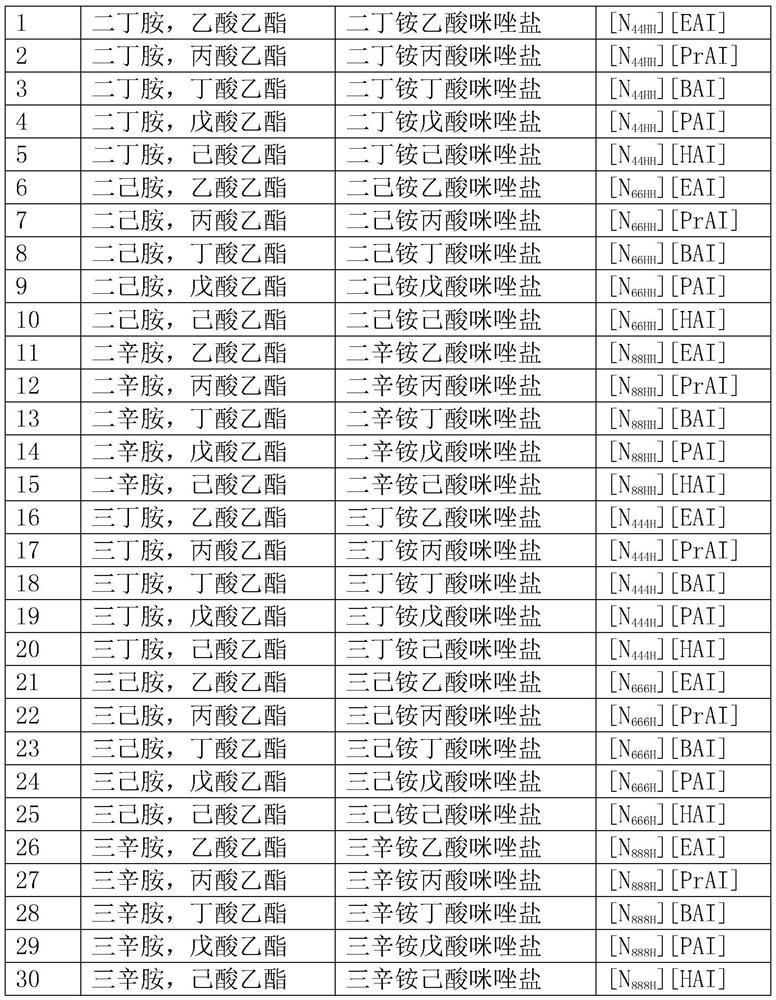
Carboxyl imidazole proton type ionic liquid as well as preparation method and application thereof
A technology of ionic liquid and salt carboxylimidazole, which is applied in the field of carboxylimidazole proton-type ionic liquid and its preparation, can solve the problems of soil pollution and water eutrophication, etc., and achieve strong structural novelty, excellent anti-friction and anti-wear properties, and raw materials Inexpensive and easy to get effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) First, dissolve imidazole, potassium hydroxide, potassium carbonate and tetrabutylammonium bromide in a round-bottomed flask filled with dichloromethane, and stir at room temperature for 2 hours. Then, ethyl acetate was slowly added dropwise into the dichloromethane system, and refluxed at 25°C for 10h. After filtering and washing with saturated saline for several times to obtain a neutral solution, and drying with anhydrous sodium sulfate, the organic phase was distilled off under reduced pressure at room temperature to obtain an oily substance which is ethyl acetate imidazole. Then, ethyl acetate imidazole was dissolved in distilled water, and the pH value of the solution was adjusted to 1 by adding concentrated sulfuric acid dropwise, and refluxed at 60°C for 10 hours. After cooling, the concentrated sulfuric acid was removed with barium chloride, and the moisture was removed by distillation under reduced pressure, that is Can get imidazole acetate;
[0034] (2)...
Embodiment 2
[0036] (1) First, dissolve imidazole, potassium hydroxide, potassium carbonate and tetrabutylammonium bromide in a round-bottomed flask filled with dichloromethane, and stir at room temperature for 4 hours. Then, ethyl hexanoate was slowly added dropwise into the dichloromethane system, and refluxed at 35°C for 15h. After filtering and washing with saturated saline for several times to obtain a neutral solution, and drying with anhydrous sodium sulfate, the organic phase was distilled off under reduced pressure at room temperature to obtain an oily substance which is ethyl imidazole hexanoate. Then, ethyl caproate imidazole was dissolved in distilled water, and the pH value of the solution was adjusted to 1 by adding concentrated sulfuric acid dropwise, and refluxed at 90°C for 15 hours. After cooling, the concentrated sulfuric acid was removed with barium chloride, and the water was removed by distillation under reduced pressure. Imidazole hexanoate can be obtained;
[0037]...
Embodiment 3
[0039] (1) First, dissolve imidazole, potassium hydroxide, potassium carbonate and tetrabutylammonium bromide in a round-bottomed flask filled with dichloromethane, and stir at room temperature for 2 hours. Then, ethyl acetate was slowly added dropwise into the dichloromethane system, and refluxed at 25°C for 10h. After filtering and washing with saturated saline for several times to obtain a neutral solution, and drying with anhydrous sodium sulfate, the organic phase was distilled off under reduced pressure at room temperature to obtain an oily substance which is ethyl acetate imidazole. Then, ethyl acetate imidazole was dissolved in distilled water, and the pH value of the solution was adjusted to 1 by adding concentrated sulfuric acid dropwise, and refluxed at 60°C for 10 hours. After cooling, the concentrated sulfuric acid was removed with barium chloride, and the moisture was removed by distillation under reduced pressure, that is Can get imidazole acetate;
[0040] (2)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com