Cefpodoxime proxetil impurity cefpodoxime dipivoxil and preparation method thereof
A technology of cefpodoxime axetil and cefpodoxime, which is applied in the field of drug impurity synthesis, can solve the problems that the structure is not listed in the Pharmacopoeia, and achieve the effects of simplified operation, high yield, and improved purity and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
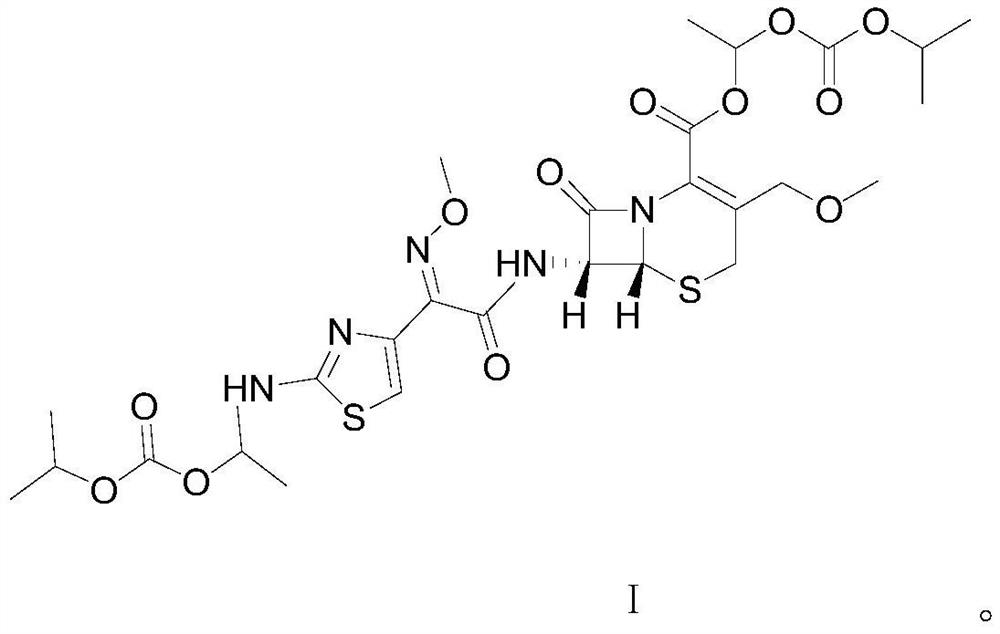
[0028] The structural formula of this cefpodoxime dipivoxil is shown in the following formula I compound:
[0029]
[0030] The concrete preparation method of above-mentioned new impurity cefpodoxime dipivoxil can adopt following method to obtain:
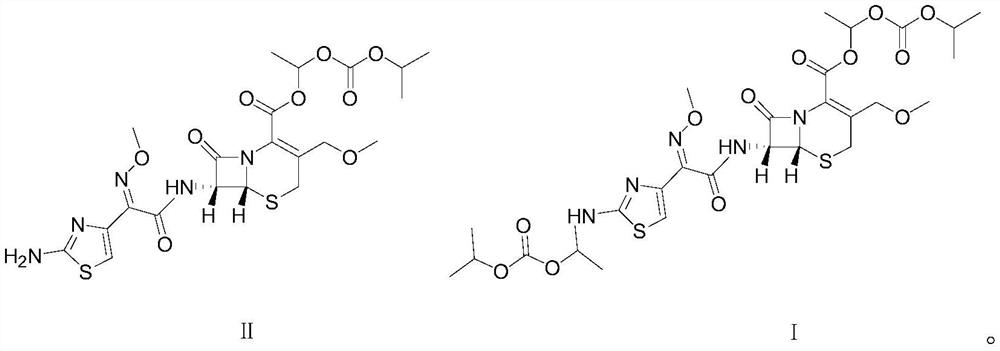
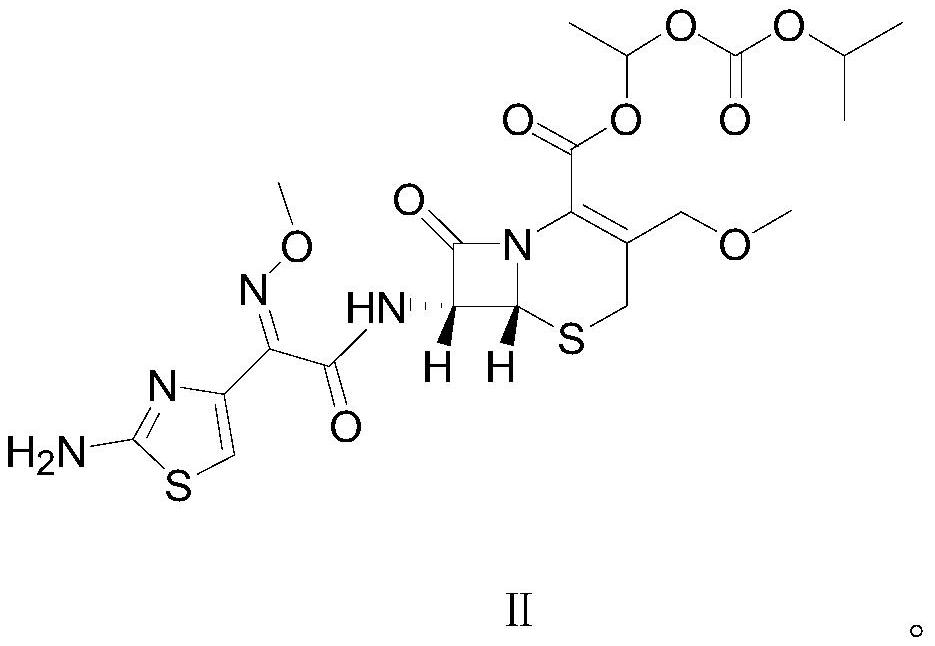
[0031] In a clean reaction flask, 5.58g (0.01mol, 1eq) of cefpodoxime axetil shown in formula II was dissolved in 72g of dichloromethane, and then 6.45g (0.025mol, 2.5eq) of 1-iodoethyliso Propyl carbonate, after stirring evenly, heat up and control the temperature at 30°C to 35°C, add 3.54g (0.035mol, 3.5eq) triethylamine dropwise, the pH of the system is about 9.0, and keep the temperature at 30°C to 35°C Condensation reaction was carried out at 0.5 ℃ for 0.5 hours. After the reaction, the temperature of the reaction solution was lowered and controlled at 5-15°C, and 40mL of dilute hydrochloric acid aqueous solution with a mass percentage of 10% was added to make the pH value about 3.4. Stirring was continued for 15 minutes, a...
Embodiment 2
[0037] The structural formula of this cefpodoxime dipivoxil is shown in the following formula I compound:
[0038]
[0039] The concrete preparation method of above-mentioned new impurity cefpodoxime dipivoxil can adopt following method to obtain:
[0040] In a clean reaction flask, 5.58g (0.01mol, 1eq) of raw material cefpodoxime axetil as shown in formula II was dissolved in 67g of toluene, and then 5.16g (0.02mol, 2.0eq) of 1-iodoethyl isopropyl was added After stirring evenly, heat up and control the temperature at 36°C to 39°C, add 2.77g (0.038mol, 3.8eq) diethylamine dropwise, the pH value is about 9.3, and keep the temperature at 36°C to 39°C Carry out the condensation reaction for 1 hour. After the reaction is over, lower the temperature of the reaction solution and control the temperature at 0-10°C, add 60 mL of 8% by mass percent dilute hydrochloric acid aqueous solution, the pH value is about 3.5, stir for 15 minutes, and let stand to separate layers. Carry out ...
Embodiment 3
[0043] In a clean reaction flask, 5.58g (0.01mol, 1eq) of raw material cefpodoxime axetil shown in formula II was dissolved in 78g of ethyl acetate, and then 6.45g (0.025mol, 2.5eq) of 1-iodoethyl Isopropyl carbonate, after stirring evenly, raise the temperature and control the temperature at 38°C, add 3.84g (0.038mol, 3.8eq) diisopropylamine dropwise, the pH value is about 9.2, and keep the temperature at 38°C ~ 40°C The condensation reaction was carried out at 0.5 hours for 0.5 hours. After the reaction, the temperature of the reaction solution was lowered and controlled at 15°C, and the pH value of the reaction solution was adjusted to about 3.0 by adding 8% by mass percent of dilute hydrochloric acid aqueous solution, stirred for 15 minutes, and allowed to stand for stratification. , perform liquid separation and discard the water layer, then add 8% dilute hydrochloric acid aqueous solution to the organic layer to adjust the pH value of the system to about 3.5, control the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com