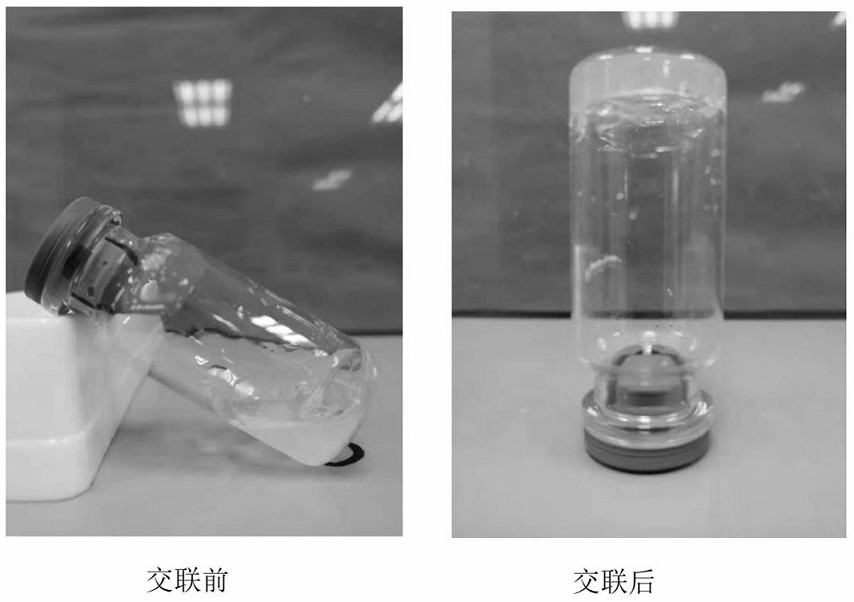
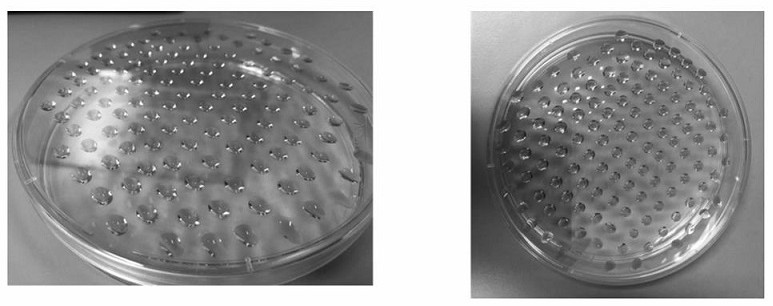
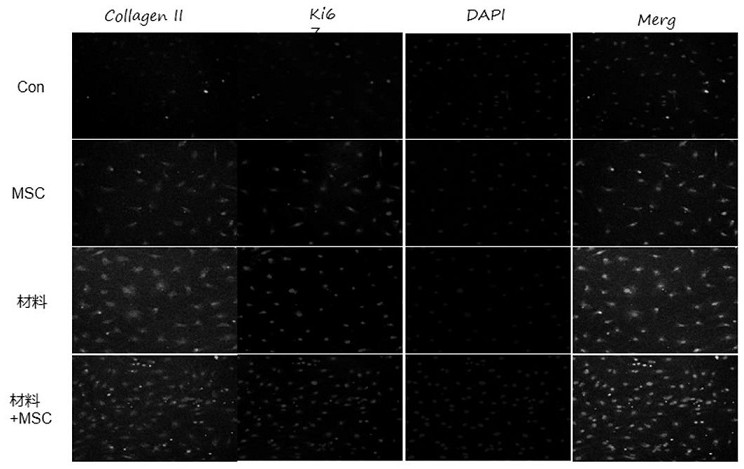
Preparation method of bi-crosslinking stem cell sphere hydrogel for osteoarthritis
A technology of stem cell spheres and osteoarthritis, applied in the fields of pharmaceutical formula, medical science, prosthesis, etc., can solve the problems of unfavorable repair of cartilage morphology and structure function, limited slow release and therapeutic effect, poor mechanical properties of hydrogel, etc. , to achieve excellent mechanical properties and biocompatibility, beneficial to the recovery of joint function, and strong anti-free radical oxidation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A preparation method for double cross-linked stem cell sphere hydrogel for osteoarthritis, specifically comprising the following steps:
[0026] (1) Add sodium carbonate to deionized water, dilute the sodium carbonate aqueous solution to adjust to pH=9, then add hyaluronic acid at a ratio of 20g / L, and then stir and dissolve at 50°C to obtain a hyaluronic acid solution. Acrylic anhydride to hyaluronic acid volume mass ratio is 3:1, N,N-dimethylformamide added in the ratio of 3ml / L, triethylamine is added in the ratio of 0.2g / L, every 12h according to Add sodium carbonate at a ratio of 1.0mmol / L, react at room temperature for 24 hours, dialyze with deionized water at room temperature for 5 days, change the water every 12 hours, the molecular weight cut-off of the dialysis bag is 8000-14000Da, and freeze-dry to obtain methyl propylene Acylated hyaluronic acid.
[0027] (2) Dissolve chondroitin sulfate in deionized water at a ratio of 10g / L to prepare chondroitin sulfate ...
Embodiment 2
[0032] A preparation method for double cross-linked stem cell sphere hydrogel for osteoarthritis, specifically comprising the following steps:
[0033](1) Add sodium carbonate to deionized water, dilute the sodium carbonate aqueous solution to adjust to pH = 10, then add hyaluronic acid at a ratio of 5g / L, and then stir and dissolve at 30°C to obtain a hyaluronic acid solution. Acrylic anhydride to hyaluronic acid volume mass ratio is 5:1, N,N-dimethylformamide added at a ratio of 5ml / L, triethylamine is added at a ratio of 0.6g / L, and pressed every 12h Add sodium carbonate at a ratio of 0.8mmol / L, react at room temperature for 40 hours, dialyze with deionized water at room temperature for 5 days, change the water every 12 hours, the molecular weight cut-off of the dialysis bag is 8000-14000Da, and freeze-dry to obtain methyl propylene Acylated hyaluronic acid.
[0034] (2) Dissolve chondroitin sulfate in deionized water at a ratio of 2g / L to prepare chondroitin sulfate solut...
Embodiment 3
[0038] A preparation method for double cross-linked stem cell sphere hydrogel for osteoarthritis, specifically comprising the following steps:
[0039] (1) Add sodium carbonate to deionized water, dilute the aqueous sodium carbonate solution to pH = 7, then add hyaluronic acid at a ratio of 10g / L, then stir and dissolve at 55°C to obtain a hyaluronic acid solution. Acrylic anhydride to hyaluronic acid volume mass ratio is 1:1, N,N-dimethylformamide is added at a ratio of 1ml / L, triethylamine is added at a ratio of 0.2g / L, and every 12h is pressed Add sodium carbonate at a ratio of 0.2mmol / L, react at room temperature for 18 hours, dialyze with deionized water at room temperature for 5 days, change the water every 12 hours, the molecular weight cut-off of the dialysis bag is 8000-14000Da, and freeze-dry to obtain methyl propylene Acylated hyaluronic acid.
[0040] (2) Dissolve chondroitin sulfate in deionized water at a ratio of 20g / L to prepare chondroitin sulfate solution, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com