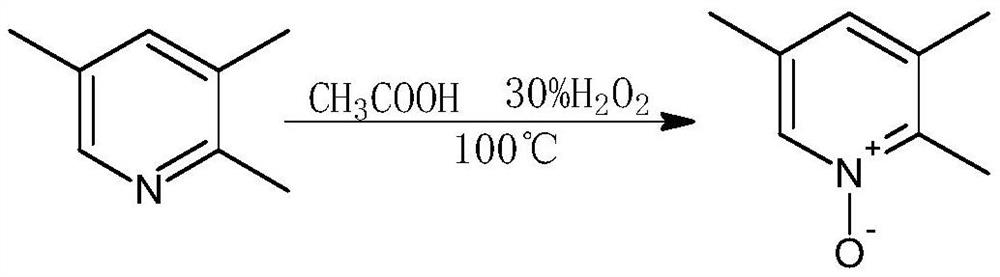Production method of omeprazole drug intermediate 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride
A technology of methoxypyridine hydrochloride and methoxypyridine, which is applied in the field of chemical synthesis, can solve the problems of many by-products, unguaranteed product quality, and difficulty in realization, so as to reduce the generation of by-products and effectively Conducive to the effect of process safety and product quality safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
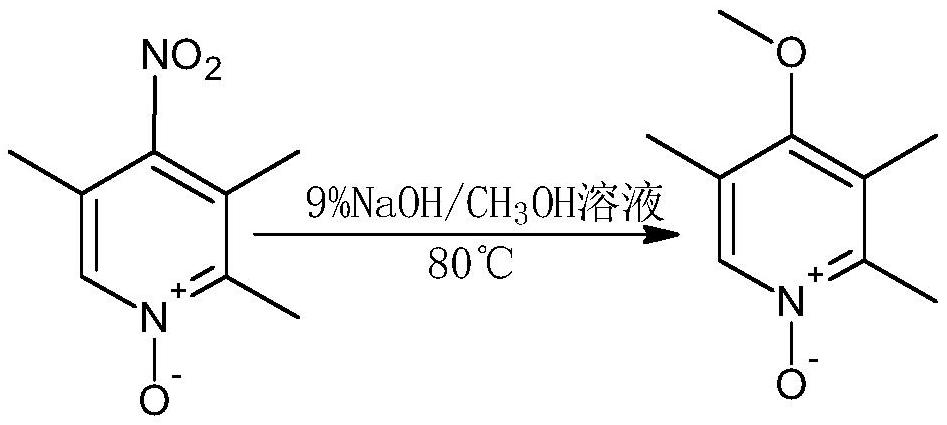
[0036] The method for the omeprazole drug intermediate 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride, the specific operations are as follows:
[0037] 1. Preparation of 2,3,5-collidine-N-oxide:
[0038] Accurately weigh raw materials 2,3,5-collidine: 300g, acetic acid: 1L, 30 270ml of % hydrogen peroxide: Put it into a dry and clean reaction kettle at a temperature of 100°C, stir for 3.2 hours, then add 30% according to the ratio of (2,3,5-collidine: 30% hydrogen peroxide = 1g: 0.5ml) Hydrogen peroxide 150ml, continue to keep stirring at 100°C for 9.1h, after the reaction is over, concentrate under reduced pressure to recover acetic acid (recycled), recrystallize the concentrate with acetone:petroleum ether=2:3, filter the mother liquor through repeated recrystallization, The final crystals were combined and dried to obtain 292 g of 2,3,5-collidine-N-oxide, with a content of 98.3% and a yield of 95.7%;
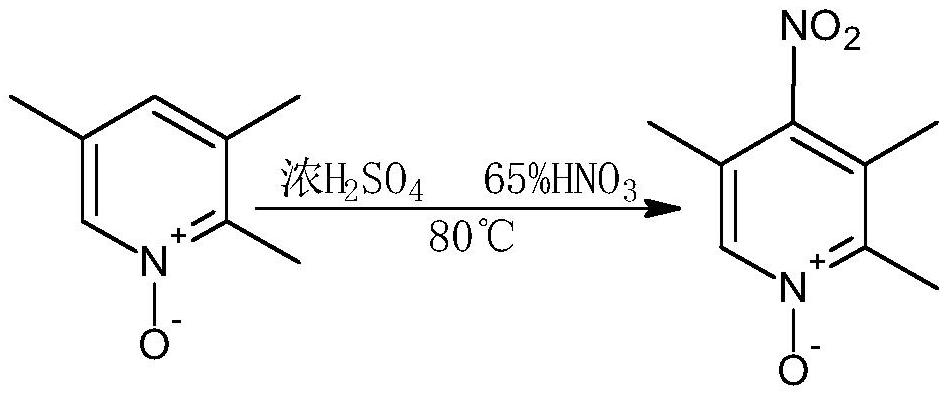
[0039] 2. Preparation of 2,3,5-trimethyl-4-nitropyridine-...
Embodiment 2
[0048] The method for the omeprazole drug intermediate 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride, the specific operations are as follows:
[0049] 1. Preparation of 2,3,5-collidine-N-oxide:
[0050] Accurately weigh raw materials 2,3,5-collidine: 400g, acetic acid: 1.3L, 30% hydrogen peroxide 360ml: Put it into a dry and clean reaction kettle at a temperature of 100°C, stir for 3.4 hours and then add 30 % hydrogen peroxide 200ml, continue to keep stirring at 100°C for 9.3 hours, after the reaction is over, concentrate under reduced pressure to recover acetic acid (recycled), recrystallize the concentrate with acetone:petroleum ether=2:3, filter to obtain the mother liquor through repeated recrystallization , the final crystals were combined and dried to obtain 390 g of 2,3,5-collidine-N-oxide, with a content of 98.2% and a yield of 95.7%;
[0051] 2. Preparation of 2,3,5-trimethyl-4-nitropyridine-N-oxide:
[0052] Accurately weigh 2,3,5-collidine-N-oxide: ...
Embodiment 3
[0060] The method for the omeprazole drug intermediate 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride, the specific operations are as follows:
[0061] 1. Preparation of 2,3,5-collidine-N-oxide:
[0062] Accurately weigh raw materials 2,3,5-collidine: 600g, acetic acid: 2L, 30 % Hydrogen peroxide 540ml: put it into a dry and clean reaction kettle, temperature 100°C, stir for 3.2 hours, then add 30% according to the ratio of (2,3,5-collidine: 30% hydrogen peroxide = 1g: 0.5ml) Hydrogen peroxide 300ml, continue to keep stirring at 100°C for 9.7h, after the reaction is over, concentrate under reduced pressure to recover acetic acid (recycled), recrystallize the concentrate with acetone:petroleum ether=2:3, filter the mother liquor through repeated recrystallization, The final crystals were combined and dried to obtain 590 g of 2,3,5-collidine-N-oxide, with a content of 97.9% and a yield of 96.2%;
[0063] 2. Preparation of 2,3,5-trimethyl-4-nitropyridine-N-oxide:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com