Single-photon up-conversion pentamethine cyanine photosensitive dye as well as preparation method and application thereof
A technology for pentamethyl tetracyanine and photosensitizing dyes, which is applied in the field of single photon up-conversion pentamethyl tetracyanine photosensitive dyes, and can solve the problem of limited quantum yield of reactive oxygen species.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1. Synthesis of photosensitive molecule XAN-Cy5:
[0059]
[0060] (1) Synthesis of intermediate compound 3:
[0061] Compound 2 (0.34g, 1mmol), quaternary ammonium salt 1 (0.63g, 2mmol) and anhydrous sodium acetate (0.2g, 2.5mmol) in absolute ethanol were stirred under reflux for 2h. After cooling, the ethanol was spin-dried by a rotary evaporator, and the crude product was purified by silica gel column chromatography (dichloromethane / methanol=10:1, v / v) to obtain compound 3 as a blue powder (0.53 g, 71.8%). 1 H NMR (400MHz, MeOD): 8.41 (d, J = 13.3Hz, 2H, CH), 7.56 (d, J = 7.4Hz, 2H, ArH), 7.46 (t, J = 7.6Hz, 2H, ArH), 7.40(d, J=7.8Hz, 2H, ArH), 7.33(t, J=7.4Hz, 2H, ArH), 6.49(d, J=13.3Hz, 2H, CH), 4.24(q, J=7.2Hz ,4H,CH 2 ),1.76(s,12H,CH 3 ), 1.45(t,J=7.2Hz,6H,CH 3 ).ESI-MS (C 29 h 34 BrN 2 I) m / z: [M–I] - calcd.489.19; found, 489.28.
[0062] (2) Synthesis of photosensitive molecule XAN-Cy5:
[0063] Dissolve 3 (0.1g, 0.16mmol), 4 (0.084g, ...
Embodiment 2
[0064] Example 2. Singlet oxygen performance test of photosensitive molecule XAN-Cy5
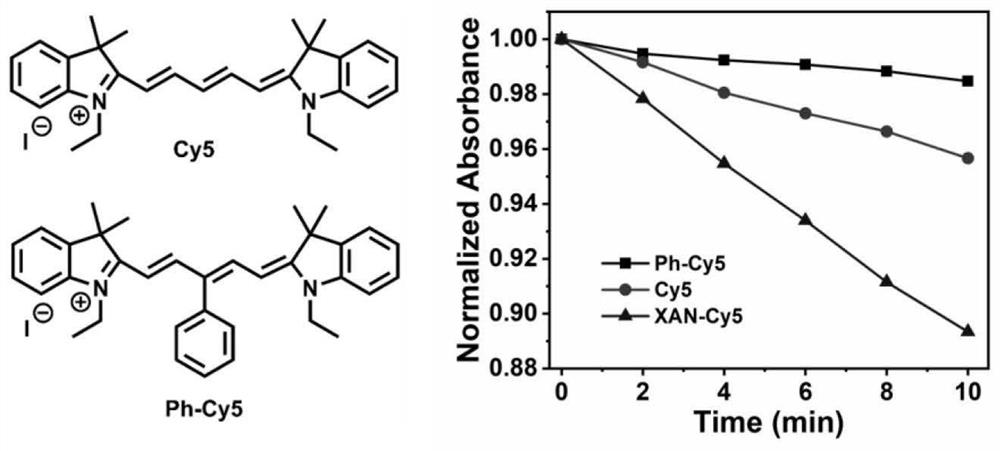
[0065] Add 2μM photosensitive molecule XAN-Cy5 to the test quartz dish containing 3mL dichloromethane solution, adjust the absorbance at 415nm to about 1 through DPBF solution, place the dish at 760nm, 500mW / cm 2 The absorption spectrum of the solution was recorded every 120 seconds. The test results are collated and displayed in figure 1 In , the absorption of the solution decays at a certain value with the increase of the irradiation time, indicating that the solution produces singlet oxygen under the irradiation of the light source of this wavelength.
Embodiment 3
[0066] Example 3. XAN-Cy5 up-conversion excitation fluorescence test
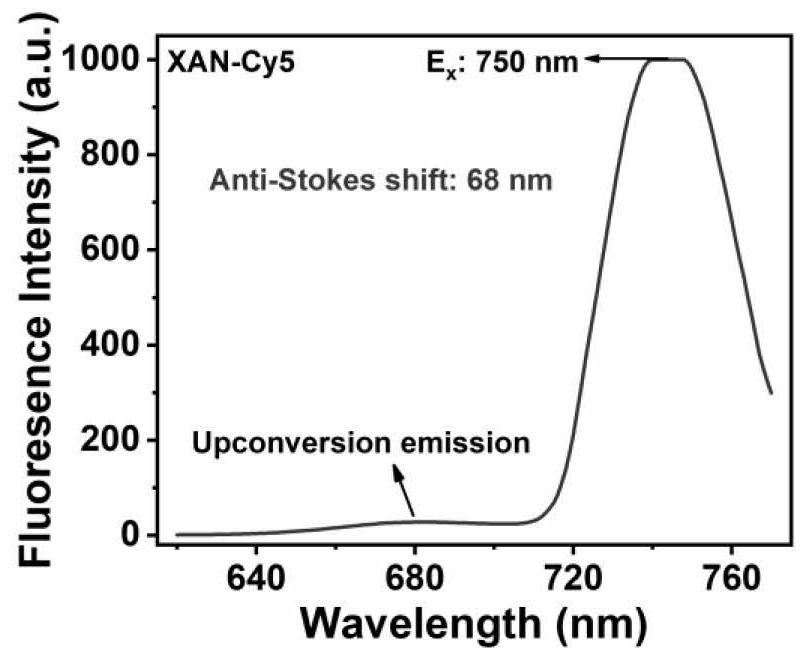
[0067] 2 μM of compound XAN-Cy5 was placed in 3 mL of dichloromethane solution. Excitation at 760nm is used and fluorescence emission at 630-700nm is captured. Such as figure 2 is the upconversion fluorescence excitation map of the photosensitive molecule XAN-Cy5
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com