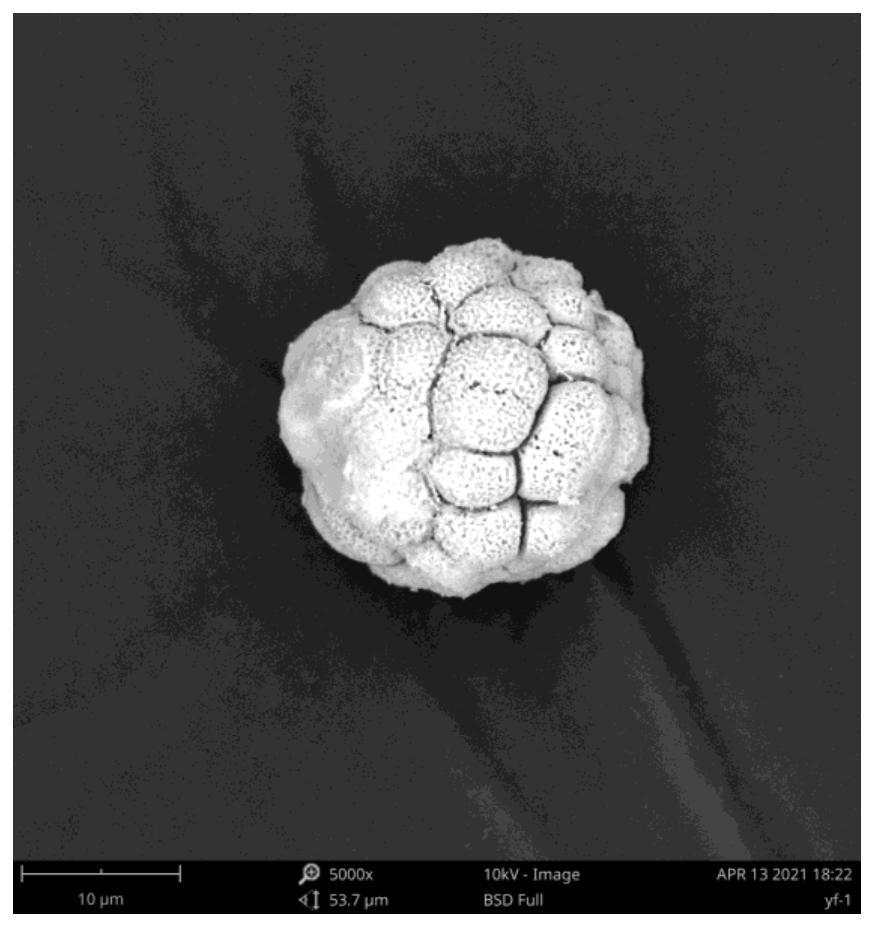
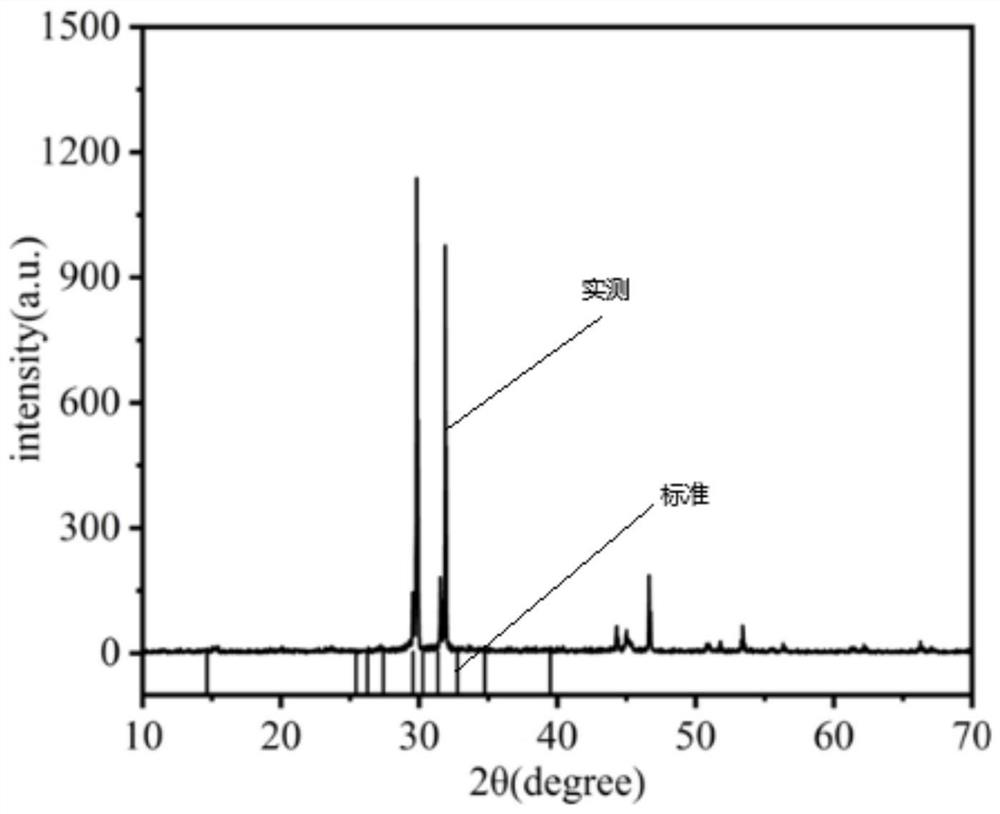
Method for preparing spherical dinitroso diammineplatinum by hydrothermal method and application
A technology of dinitrosodiammine platinum and hydrothermal method is applied in the field of preparing spherical dinitrosodiammine platinum by hydrothermal method, which can solve the problem of structural instability, uneven morphology of P salt, affecting product plating quality, etc. problem, to achieve the effect of good electroplating quality, convenient operation method and good crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of spherical dinitrosodiammine platinum provided by the invention comprises the following steps:
[0033] 1) Precipitation of potassium chloroplatinate is prepared by reacting chloroplatinic acid with potassium chloride;
[0034] 2) Sodium nitrite reduction substitution:
[0035] Sodium nitrite is used as a reducing agent to reduce potassium chloroplatinate. The process is to dissolve the precipitated potassium chloroplatinate in water, add sodium nitrite solution for reduction after heating, no yellow-brown gas is produced in the final reaction, and the solution is transparent yellow-green, natural After cooling to room temperature, filter;
[0036] The specific method is to dissolve the potassium chloroplatinate precipitate in water, heat the oil bath to 85-100°C, add the sodium nitrite solution dropwise, control the reaction system at 100-110°C, and continue stirring for 1.5-2 hours after the dropwise addition , to obtain a yellow-green trans...
Embodiment 1
[0055] 1) Precipitation of potassium chloroplatinate by reaction of chloroplatinic acid and potassium chloride
[0056] 1.1) Take 50g of chloroplatinic acid solids in a beaker with an electronic balance, and prepare a chloroplatinic acid solution with a mass fraction of 10%;
[0057] 1.2) Weigh 1.5g of potassium chloride solid in a beaker, add 15ml of pure aqueous solution to obtain a 0.1g / ml potassium chloride solution;
[0058]1.3) Transfer 15ml potassium chloride solution to a 250ml three-neck flask, heat the oil bath to 90°C, slowly add 50ml of the prepared 10% potassium chloroplatinate solution dropwise, continue stirring for 1 hour after the dropwise addition, and cool to 5°C , filtered with suction and washed with ice-pure water, dried at 80°C, and the product was a yellow precipitate of potassium chloroplatinate;
[0059] 2) In a 250ml flask, 2.5g of potassium chloroplatinate precipitate was mixed with 5ml of pure water to make a paste; the oil bath was heated until t...
Embodiment 2
[0064] 1.1) Take 50g of chloroplatinic acid solids in a beaker with an electronic balance, and prepare a chloroplatinic acid solution with a mass fraction of 10%;
[0065] 1.2) Weigh 3g of potassium chloride solid in a beaker, add 30ml of pure aqueous solution to obtain 30ml of potassium chloride solution;
[0066] 1.3) Transfer 30ml of potassium chloride solution to a 250ml three-necked flask, heat the oil bath to 85°C, slowly add 100ml of the prepared 10% potassium chloroplatinate solution dropwise, continue stirring for 1 hour after the dropwise addition, and cool to 0°C , suction filtered and washed with ice-pure water, dried at 85°C, and the product was a yellow precipitate of potassium chloroplatinate;
[0067] 2) In a 250ml flask, mix 5g of potassium chloroplatinate solid with 10ml of pure water to make a paste; heat the oil bath until the reaction system is 85°C, start adding 50ml of the prepared sodium nitrite solution dropwise, and control the reaction system to 110°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com