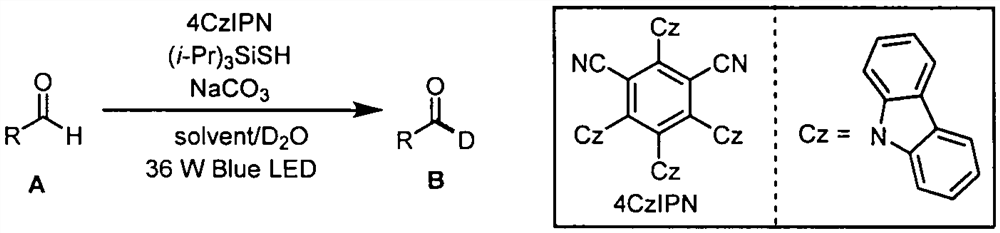
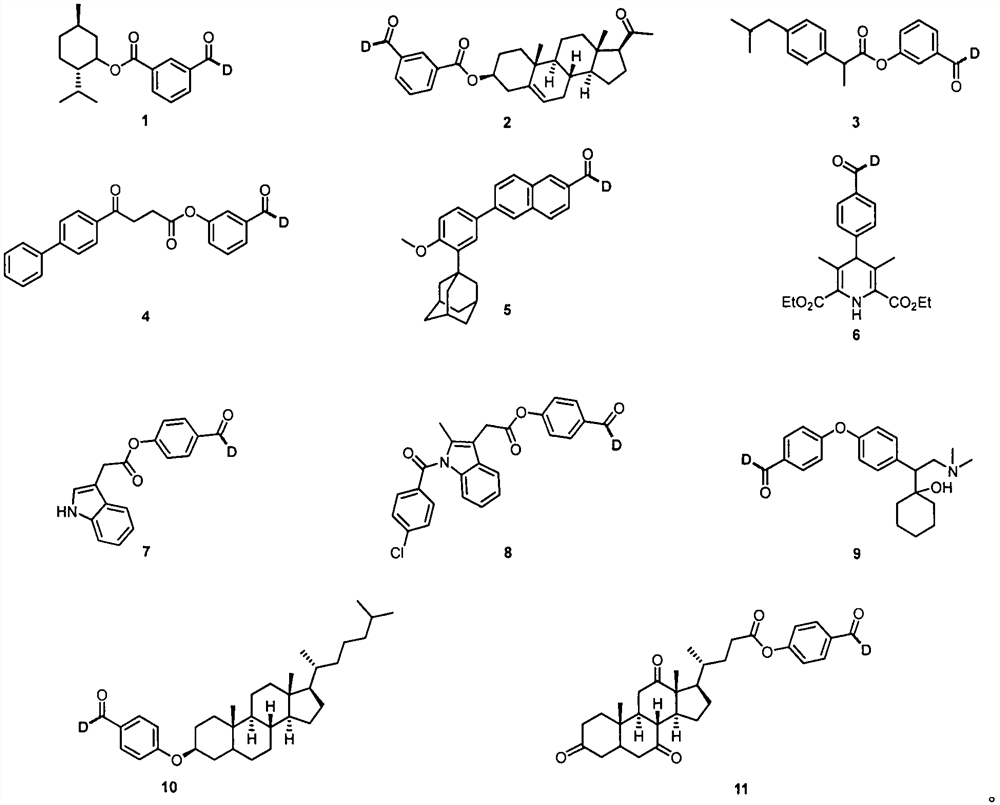
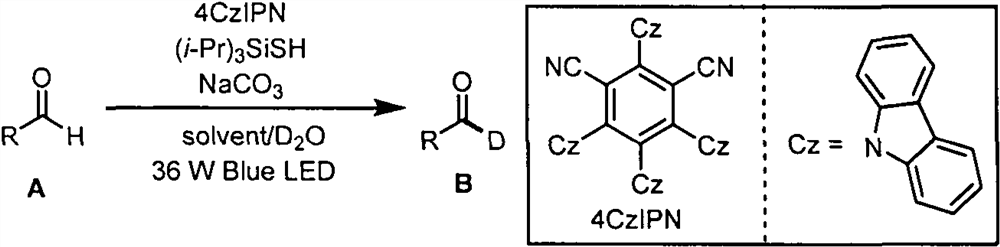
Aldehyde deuteration and application of aldehyde deuteration in preparation of deuterated aldehyde
A technology of deuterated and deuterated water, applied in the application field of the preparation of deuterated aldehydes, can solve the problems of low deuteration rate and poor regioselectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Synthesis of (2S, 5R)-2-isopropyl-5-methylcyclohexyl 3-formylbenzoate-formyl-d1:
[0024] Weigh 0.2mmol (2S, 5R)-2-isopropyl-5-methylcyclohexyl 3 formyl benzoate, 0.01mmol photocatalyst 4CzIPN, 0.08mmol triisopropylsilyl mercaptan ((i- Pr) 3 SiSH), 0.08mmol sodium carbonate (NaCO 3 ) in an 8mL reaction flask, then add 1mL ethyl acetate solution, 1mL deuterated water, blow argon for 30s in the reaction flask, react under 36W 470nm blue light irradiation for 36 hours, spin off the solvent, column chromatography (petroleum ether : ethyl acetate=20:1) to obtain a yellow liquid with a yield of 88% and a deuterated rate of 91%. 1 H NMR (400MHz, CDCl 3 )δ10.10(s, 0.09H), 8.53(s, 1H), 8.32(d, J=7.6Hz, 1H), 8.09(d, J=7.6Hz, 1H), 7.64(t, J=7.6Hz , 1H), 5.00(td, J=10.8, 4.4Hz, 1H), 2.13(d, J=12.0Hz, 1H), 1.95(dtd, J=13.6, 6.8, 2.4Hz, 1H), 1.80-1.70( m, 2H), 1.65-1.51(m, 2H), 1.14(dd, J=23.2, 11.6Hz, 2H), 1.03-0.88(m, 7H), 0.81(d, J=6.8Hz, 3H). 13 C NMR (100MHz, CD...
Embodiment 2
[0025] Example 2: Synthesis of 3-(formyl-d)phenyl 2-(4-isobutylphenyl)propionate:
[0026] Weigh 0.2mmol 3-formylphenyl 2-(4-isobutylphenyl) propionate, 0.01mmol photocatalyst 4CzIPN, 0.08mmol triisopropylsilyl mercaptan ((i-Pr) 3 SiSH), 0.08mmol sodium carbonate (NaCO 3 ) in an 8mL reaction flask, then add 1mL ethyl acetate solution, 1mL deuterated water, blow argon for 30s in the reaction flask, react for 36 hours under 36W 470nm blue light irradiation, spin off the solvent, column chromatography (petroleum ether : ethyl acetate=20:1) to obtain a yellow liquid with a yield of 80% and a deuterated rate of 93%. 1 H NMR (400MHz, CDCl 3)δ9.95(s, 0.07H), 7.71(d, J=7.6Hz, 1H), 7.51(dt, J=15.6, 4.8Hz, 2H), 7.35-7.23(m, 3H), 7.16(t, J=6.4Hz, 2H), 3.96(q, J=7.2Hz, 1H), 2.47(d, J=7.2Hz, 2H), 1.87(tt, J=13.2, 6.8Hz, 1H), 1.61(d, J=7.2Hz, 3H), 0.91(d, J=6.8Hz, 6H). 13 C NMR (100MHz, CDCl 3 )δ191.0 (t, J=27.5Hz), 173.0, 151.5, 141.1, 137.6 (t, J=3.5Hz), 136.9, 130.1, 129.7, 127.8, ...
Embodiment 3
[0027] Example 3: Synthesis of 6-(3-((3r, 5r, 7r)-adamantan-1-yl)-4-methoxyphenyl)-2 naphthaldehyde-d1:
[0028] Weigh 0.2mmol 6-(3-((3r,5r,7r)-adamantan-1-yl)-4-methoxyphenyl)-2 naphthaldehyde, 0.01mmol photocatalyst 4CzIPN, 0.08mmol triisopropyl Silylthiol ((i-Pr) 3 SiSH), 0.08mmol sodium carbonate (NaCO 3 ) in an 8mL reaction flask, then add 1mL ethyl acetate solution, 1mL deuterated water, blow argon for 30s in the reaction flask, react for 36 hours under 36W 470nm blue light irradiation, spin off the solvent, column chromatography (petroleum ether : ethyl acetate=20:1) to obtain a white solid with a yield of 82%, a deuterated rate of 96%, and a melting point of 236-237°C. 1 H NMR (400MHz, CDCl 3 ( d, J=2.4Hz, 1H), 7.56(dd, J=8.4, 2.4Hz, 1H), 7.01(d, J=8.4Hz, 1H), 3.91(s, 3H), 2.18(s, 6H), 2.11(s, 3H), 1.81(s, 6H). 13 C NMR (100MHz, CDCl 3 )δ 191.9 (t, J=27Hz), 159.1, 142.3, 139.1, 136.9, 134.3, 133.7 (t, J=3.1Hz), 132.3, 131.4, 131.3, 129.9, 129.8, 129.2, 126.9, 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com