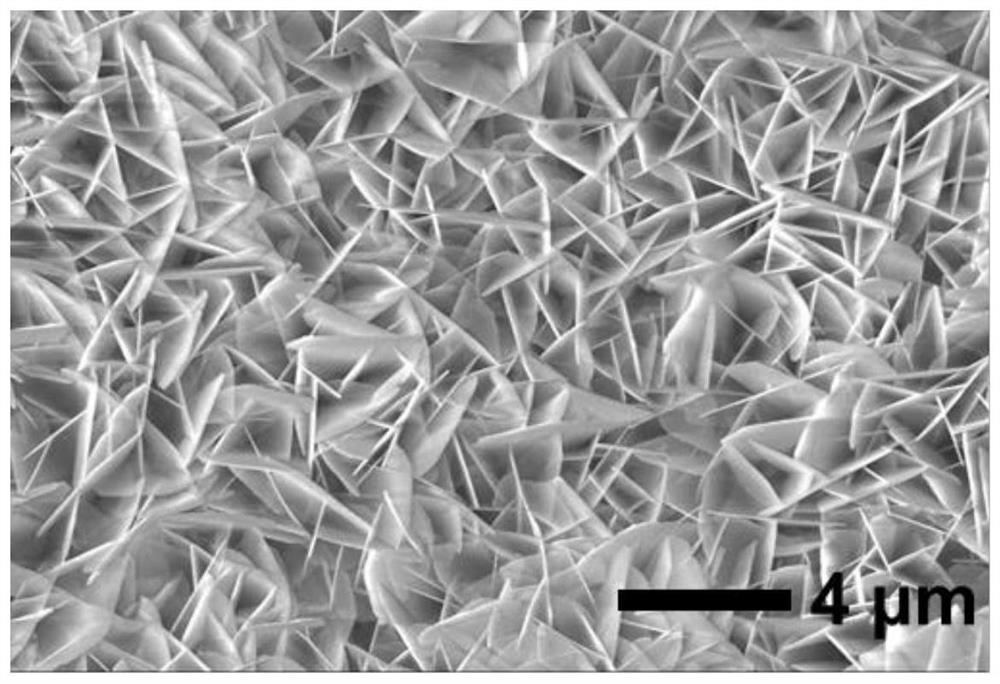
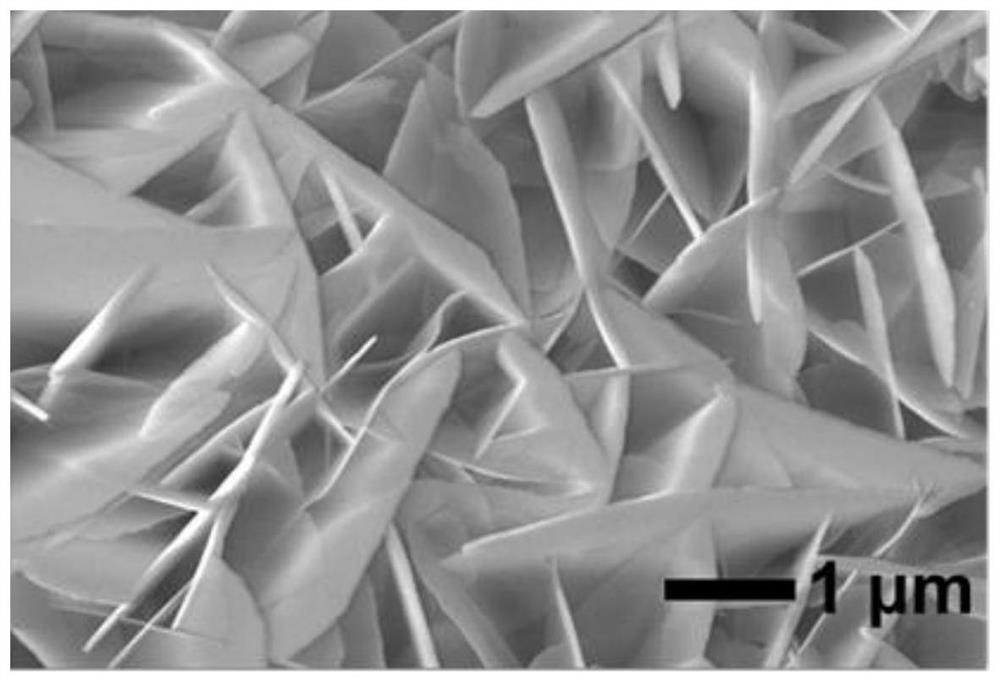
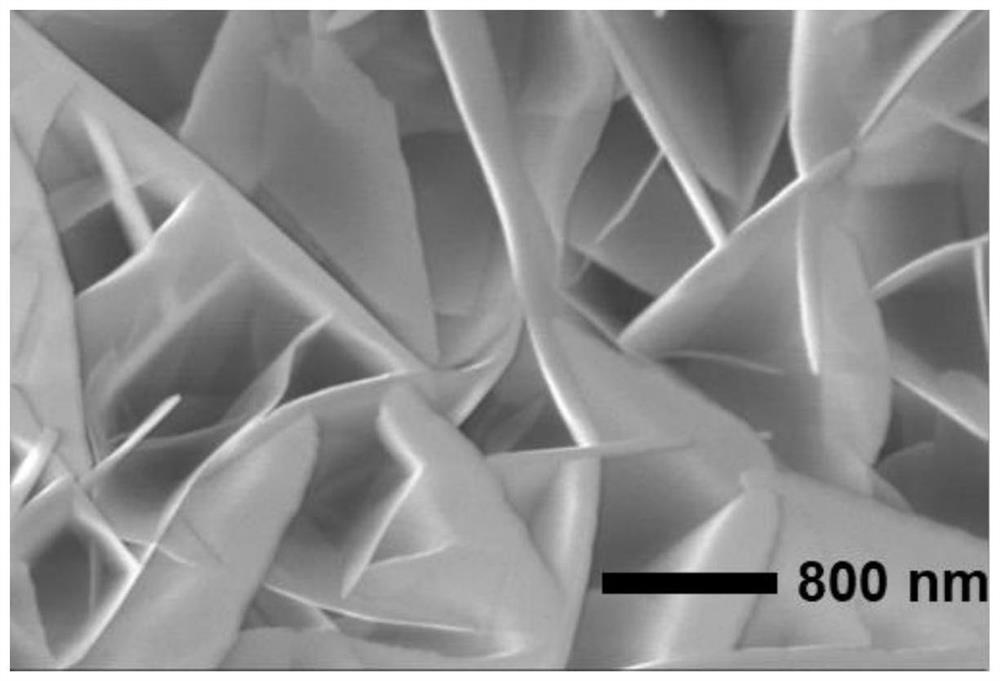
Non-noble metal catalyst for glycerin oxidation assisted hydrogen production
A non-precious metal and catalyst technology, applied in the field of materials, can solve the problems of restricting large-scale production, high cost, scarcity and poor stability, and achieve the effects of good charge conductivity, easy operation and low preparation cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Pretreatment of nickel foam
[0043] Cut the foamed nickel into a size of 2cm×3cm, and then put the foamed nickel cut into 3M hydrochloric acid, absolute ethanol and deionized water for 10 minutes respectively;
[0044] 2. Preparation of Co 3 o 4 @NF
[0045] Dissolve 4.365g of cobalt nitrate hexahydrate and 0.164g of 2-methylimidazole in 20ml of methanol respectively to obtain solution A and solution B. After stirring evenly, place liquid A under a magnetic stirrer, and pour liquid B into liquid A , and stirred for 3-5min to obtain solution C evenly. The solution C and the treated foamed nickel were put into a reaction vessel together, and subjected to hydrothermal reaction at 140°C for 12h, then washed with methanol and absolute ethanol three times, and vacuum-dried at 70°C for 12h to obtain a precursor. Finally, put the dried precursor in a porcelain boat, and then place it in a high-temperature tube furnace to raise the temperature to 350°C at a rate of 2°C p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com