Organic charge transfer eutectic and preparation method and application thereof
A charge-transfer, organic technology, applied in the field of organic charge-transfer eutectics and their preparation, to achieve the effects of simple preparation methods, broadened applications, and excellent photothermal conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] ABTS +· The preparation method of solution comprises the steps:
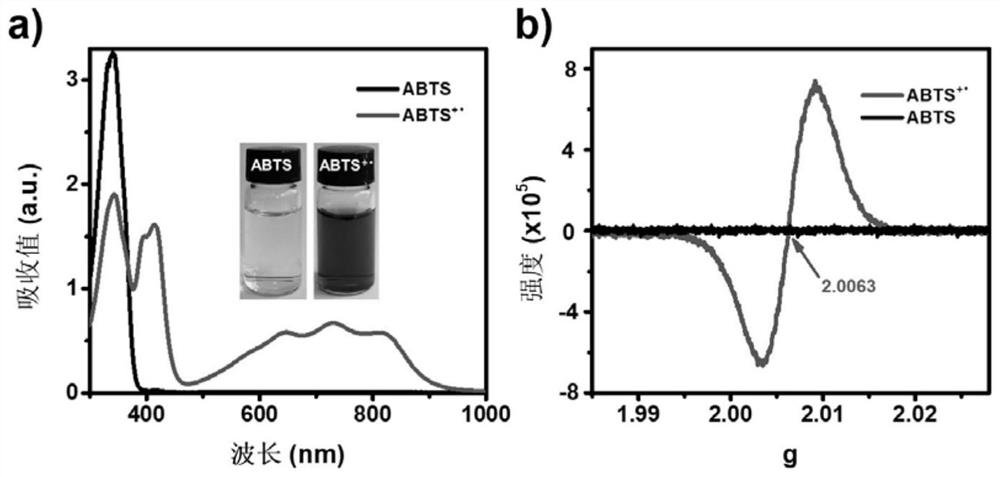
[0062] Dilute hydrochloric acid aqueous solution to obtain a weak acid solution with pH 4; use water as a solvent to prepare 50 mM negative divalent 2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS 2- ) solution, stored at 4°C; dilute the Ru@G nanoparticles to 0.1 μg / mL. In a 50mL centrifuge tube, add 39.5mL of pH 4 diluted hydrochloric acid aqueous solution, then add 300μL of 50mM ABTS 2- solution, 200 μL 1M H 2 o 2 , 40μL 100μg / mL Ru@G, shake evenly, the solution gradually changes from colorless to green, and Ru@G and ABTS +· Green mixed solution; after standing for 12 hours, collect ABTS by centrifugation +· Green solution, the centrifugal speed is 10000rpm. to ABTS +· To characterize, the resulting graph figure 1 shown; where, a) is ABTS +· The UV-Vis-NIR absorption spectrum; b) is ABTS +· electron paramagnetic resonance spectrum. It can be seen that the obtained ABTS +· The ult...
Embodiment 2
[0064] Organic charge-transfer co-crystals based on persistent cationic radicals were prepared as follows:
[0065] Collect all ABTS in embodiment 1 +· The solution was added to a 20mL serum bottle, and then 2mL of 10mM M TMB mother solution was added. The TMB mother solution was TMB dissolved in ethanol. The color changed rapidly from green to blue, and gradually formed flocculents. Finally, a large amount of blue-green eutectic precipitated at the bottom of the bottle, and the crystals were collected, and then freeze-dried to obtain organic charge transfer eutectic TAHC powder.
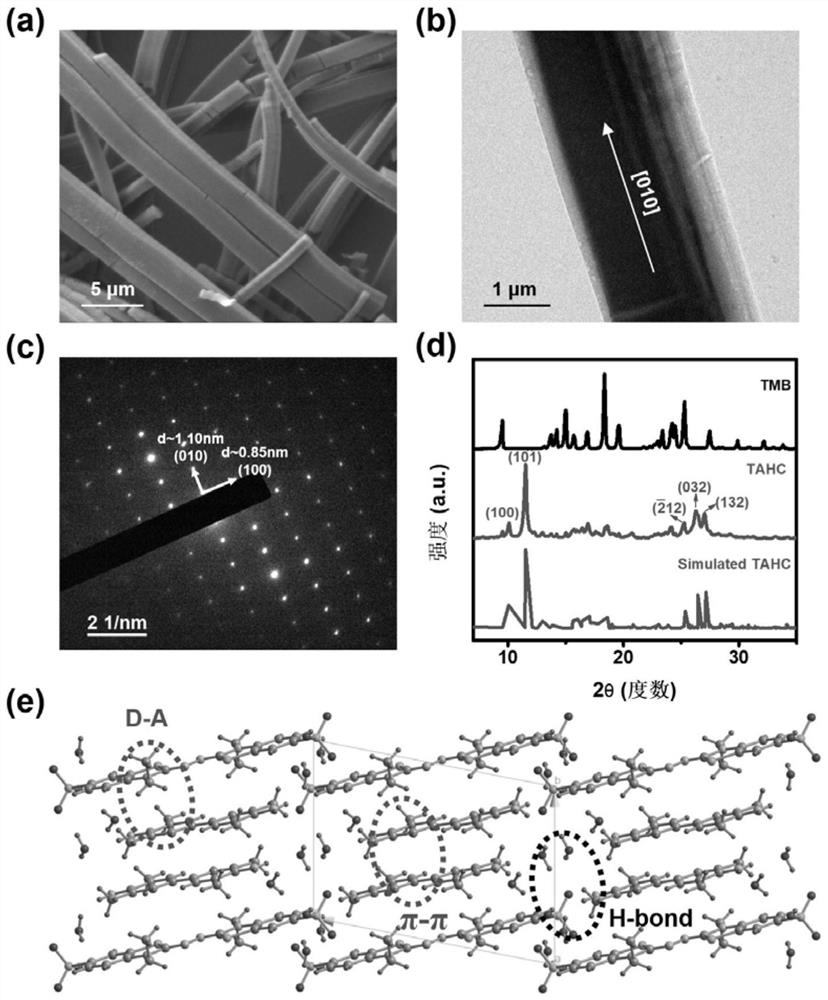
[0066] Structural elucidation of organic charge-transfer eutectic TAHC, such as figure 2 Shown, wherein a) is the SEM image of TAHC; b) is the TEM image of TAHC; c) is the selected area electron diffraction of TAHC; d) is the powder X-ray diffraction spectrum of TAHC and TMB; e) is the crystal of TAHC Structural Analysis Diagram. It can be seen that the prepared eutectic presents a one-dimension...
Embodiment 3
[0068] The preparation method of the organic charge-transfer co-crystal based on common acceptors is the same as that in Example 2, the difference is to compare the influence of different types of acceptors on the absorption properties of the charge-transfer co-crystal.
[0069] The ABTS of embodiment 2 +· The solution was changed to 1,2,4,5-tetracyanobenzene (TCNB) solution, and the mass ratio of TMB to TCNB was 1:1; a TMB-TCNB charge-transfer cocrystal (TTC) was obtained as a cationic radical acceptor control.
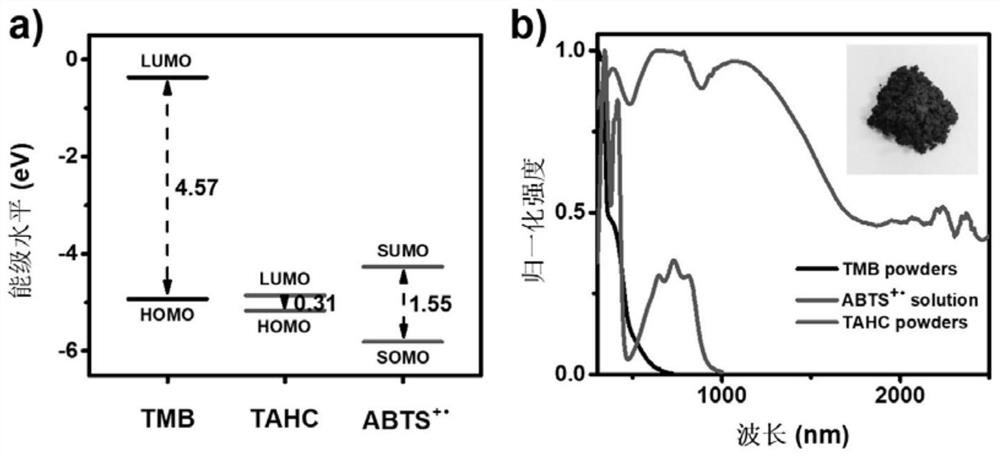
[0070] The TMB-TCNB charge-transfer eutectic was tested by UV-visible-near-infrared absorption spectrum, and the experimental results ( Figure 4 ) shows that the absorption of TTC eutectic can only reach 1000nm. It fully demonstrates that in the case of the same donor, the type of acceptor will have a huge impact on the properties of the formed charge transfer, especially the UV-Vis-NIR absorption, and then affect their photothermal performance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| photothermal conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com