Enterococcus faecalis for treating colitis
A technology for Enterococcus faecalis and colitis, applied in the field of Enterococcus faecalis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
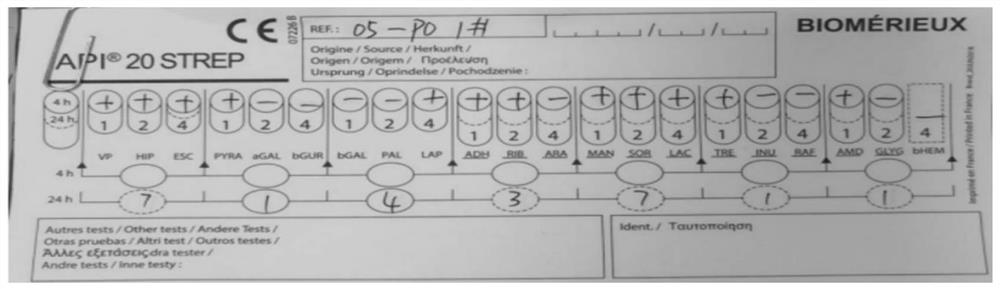
[0099] Example 1 Isolation and identification of Enterococcus faecalis
[0100] (1) Strain isolation and sequencing
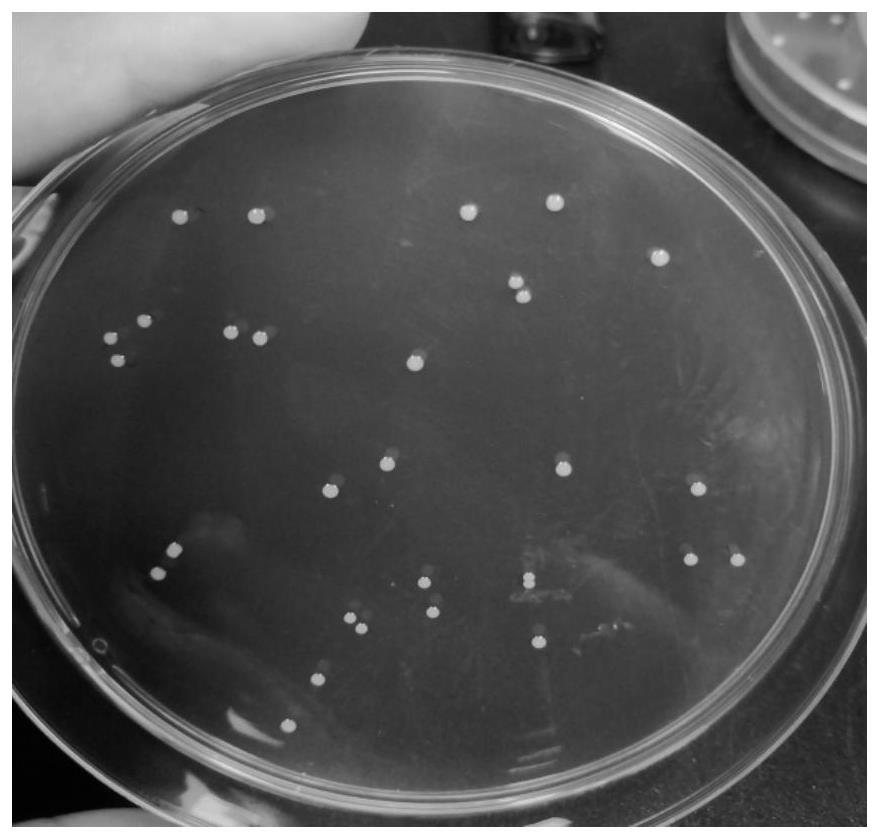
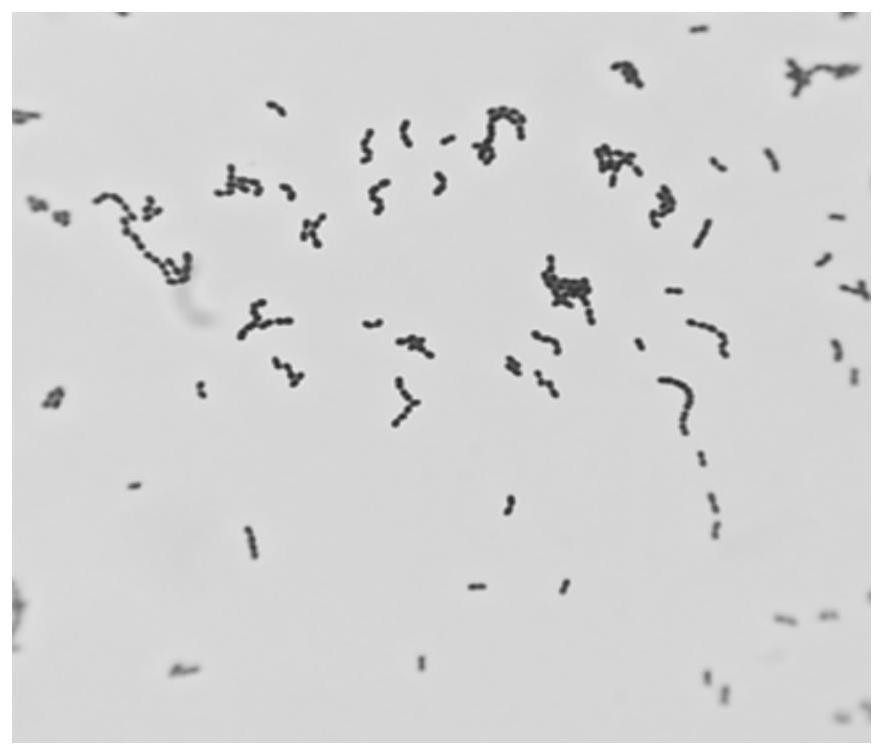
[0101] Under sterile conditions, the prepared YK-20210225 donor fecal bacteria liquid was diluted 10 times, and three concentration gradients were selected, streaked and inoculated on NA and MRS medium, and three replicate plates were incubated at a constant temperature of 37°C. Anaerobic culture in the box for 72 hours, then pick different forms of colonies for Gram staining and microscopic examination, and carry out 2 times of streaking and purification in MRS medium, then staining and microscopic examination, observe the bacterial morphology and whether it is purified, and then carry out 16S For rRNA sequencing, universal primers were used, wherein the forward primer was 27F, whose sequence was shown in SEQ ID NO.2; the reverse primer was 1429R, whose sequence was shown in SEQ ID NO.3.
[0102] The PCR reaction system is as follows:
[0103] Prem...
Embodiment 2
[0137]Example 2 Detection of Resistance to Artificial Gastrointestinal Fluid and Detection of Bile Salt Resistance
[0138] (1) MTT solution preparation: Weigh 0.5g, add 100mL PBS to dissolve (final concentration is 5mg / mL), filter and sterilize, aliquot into 15mL centrifuge tubes, freeze at -20°C for later use, valid for half a year.
[0139] (2) Simulated Gastric Fluid (SGF) experiment
[0140] 1. Take 2.0g NaCl and 3.2g pepsin (Soleibao pepsin, 1:3000, marked as 800-2500 activity units per mg), add 7.0mL 37% dilute hydrochloric acid and water to dissolve to 1000mL , namely, the pH value of the solution is 1.2;
[0141] 2. Adjust the pH to pH 1.2 (simulating the pH of the fasting intestinal fluid of a human), pH 2.0, pH 3.0 (simulating the pH of the intestinal fluid of a human after meals and the pH of the fasting intestinal fluid of a mouse) and pH 4.0, and filter to sterilize;
[0142] 3. Bacteria collection: Bacterial strains were cultured at 37°C for 8 hours, and reach...
Embodiment 3
[0166] Embodiment 3 in vitro drug effect test
[0167] LPS mother liquor configuration: dissolve in DMEM, prepare 1 mg / mL mother liquor, and store in -20°C refrigerator.
[0168] Experimental operation: RAW264.7 cell culture (DMEM high glucose medium + 10% FBS, 37°C + 5% CO 2 For culture), the cells in a better culture state are blown off the adherent cells with the culture medium (the ones that cannot be blown off can be discarded), blown evenly, and the cells are counted, and diluted to obtain a certain concentration of cell culture medium (DMEM +10% FBS), seeded in 24-well cell culture plate, the number of cells per well is about 2×10 6 per well, the inoculum volume is 250 μL. After incubation for 16 hours, set blank control group, LPS (1 μg / mL), LPS (1 μg / mL) + 10% culture supernatant of PRS-217-05 bacteria (that is, the strains were cultured in MRS liquid medium for 24 hours, and then processed by high-speed centrifugation Obtain the bacterial culture supernatant, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com