Composition capable of effectively and rapidly relieving infantile eczema sicca as well as preparation method and application of composition
A technology for infant dryness and composition, which is applied in the direction of medical preparations containing active ingredients, skin care preparations, pharmaceutical formulas, etc., which can solve uneven levels, difficulty in ensuring safety, lack of natural compositions for dry eczema, etc. problem, to achieve the effect of eliminating acne, improving desquamation, and relieving sensitive skin eczema
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
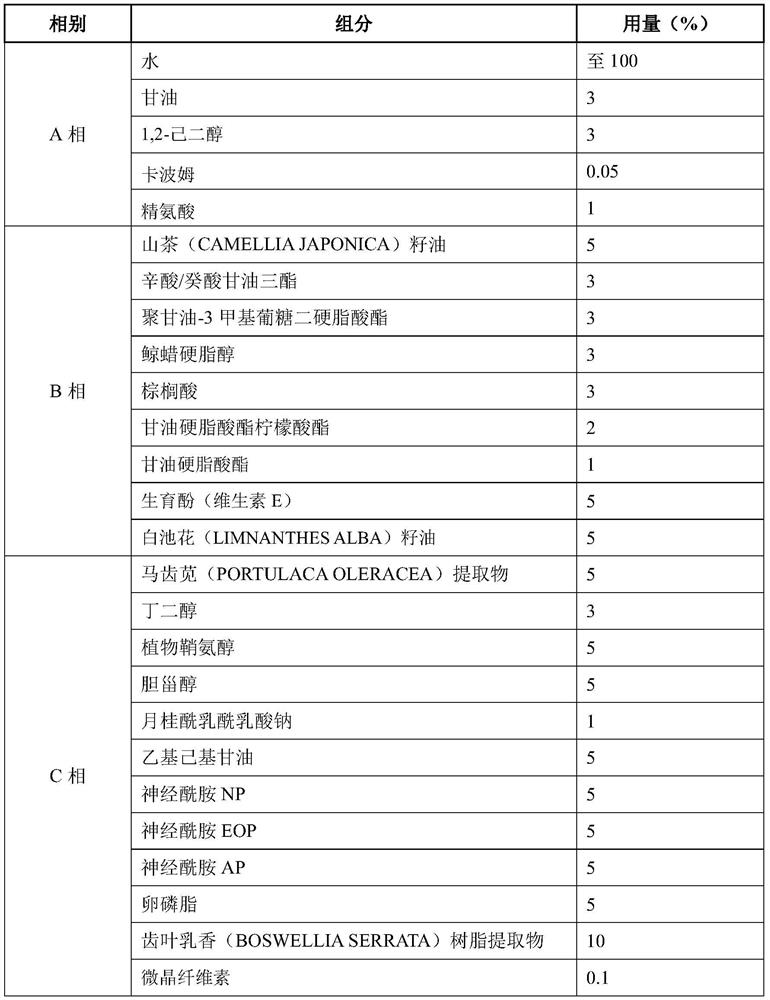
[0037] A composition for effectively and rapidly relieving infant dry eczema, the ingredients of which are shown in Table 1 below.
[0038] All raw materials meet the corresponding national standards.
[0039] Table 1. Composition ingredient list (by mass percentage).
[0040]
[0041] This embodiment also provides a preparation method of the composition for effectively and rapidly relieving infant dry eczema, comprising:
[0042] Step 1. Weigh each raw material in proportion and divide it into phase A, phase B and phase C.
[0043] Step 2. Raise the temperature of Phase A until it is completely dissolved.
[0044] Step 3. After phase B is heated to complete dissolution, add to phase A and emulsify for 5 minutes.
[0045] Step 4. After cooling the obtained mixture to 40°C, add phase C and stir evenly.
[0046] Step 5, continue to stir and cool down to room temperature to complete the preparation of the composition.
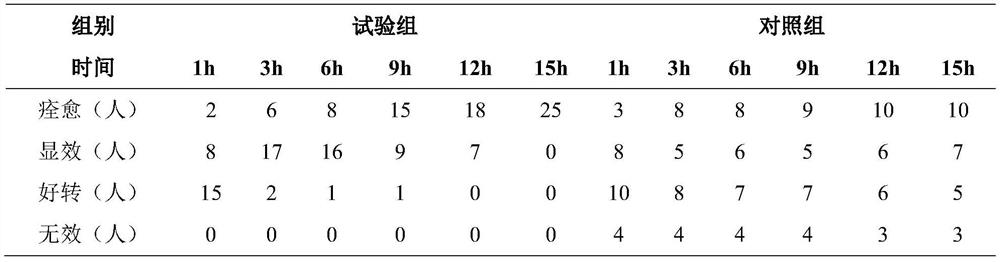
[0047] This embodiment also provides the use of the ...
Embodiment 2
[0049] A composition for effectively and rapidly relieving infant dry eczema, comprising phase A, phase B and phase C.
[0050] Preferably, the composition comprises by parts by weight:
[0051] Phase A, 2.8 parts of glycerin, 2.8 parts of 1,2-hexanediol, 0.04 parts of carbomer, and 0.8 parts of arginine.
[0052] Phase B: 4.5 parts of camellia seed oil, 2.8 parts of caprylic / capric triglyceride, 2.8 parts of polyglyceryl-3 methylglucose distearate, 2.8 parts of cetearyl alcohol, 2.8 parts of palmitic acid, glycerin stearate Fatty acid ester citrate 1.6 parts, glyceryl stearate 0.8 parts, tocopherol ie vitamin E 4.5 parts, white pond flower seed oil 4.5 parts.
[0053] Phase C, 4.5 parts of purslane extract, 2.8 parts of butanediol, 4.5 parts of phytosphingosine, 4.5 parts of cholesterol, 0.8 parts of sodium lauroyl lactylate, 4.5 parts of ethylhexylglycerin, 4.5 parts of ceramide NP, Ceramide EOP 4.5 parts, Ceramide AP 4.5 parts, Lecithin 4.5 parts, Boswellia serrata resin ...
Embodiment 3
[0063] A composition for effectively and rapidly relieving infant dry eczema, comprising phase A, phase B and phase C.
[0064] Preferably, the composition comprises by parts by weight:
[0065] Phase A, 3.3 parts of glycerin, 3.3 parts of 1,2-hexanediol, 0.08 parts of carbomer, and 1.2 parts of arginine.
[0066] Phase B: 5.4 parts of camellia seed oil, 3.3 parts of caprylic / capric triglyceride, 3.3 parts of polyglyceryl-3 methylglucose distearate, 3.3 parts of cetearyl alcohol, 3.3 parts of palmitic acid, glycerin stearate 2.2 parts of fatty acid ester citrate, 1.2 parts of glyceryl stearate, 5.4 parts of tocopherol, namely vitamin E, and 5.4 parts of white pond flower seed oil.
[0067] Phase C, 5.4 parts of purslane extract, 3.3 parts of butanediol, 5.4 parts of phytosphingosine, 5.4 parts of cholesterol, 1.2 parts of sodium lauroyl lactylate, 5.4 parts of ethylhexylglycerin, 5.4 parts of ceramide NP, Ceramide EOP 5.4 parts, Ceramide AP 5.4 parts, Lecithin 5.4 parts, Bos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com