Four engineering saccharomycetes capable of efficiently synthesizing intermediate products or end products in synthetic route of ginsenoside Ro and method for efficiently synthesizing intermediate products or end products in synthetic route of ginsenoside Ro
A technology for yeast and Panax ginseng saponins, which is applied in the fields of synthetic biology and metabolic engineering, can solve the problems of complex glycosyl modification, difficult de novo synthesis, unknown natural synthesis route, etc., and achieves the effect of efficient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
[0102] Experimental example, experimental operation of the present invention
[0103] YPD medium: about 10g / L yeast extract, 20g / L glucose, 20g / L peptone, prepare solid medium and add 20g / L agar powder.
[0104] During cultivation, use a 100mL Erlenmeyer flask, use a constant temperature shaker, 30°C, 200rpm, for cultivation, and generally cultivate for about 5 days.
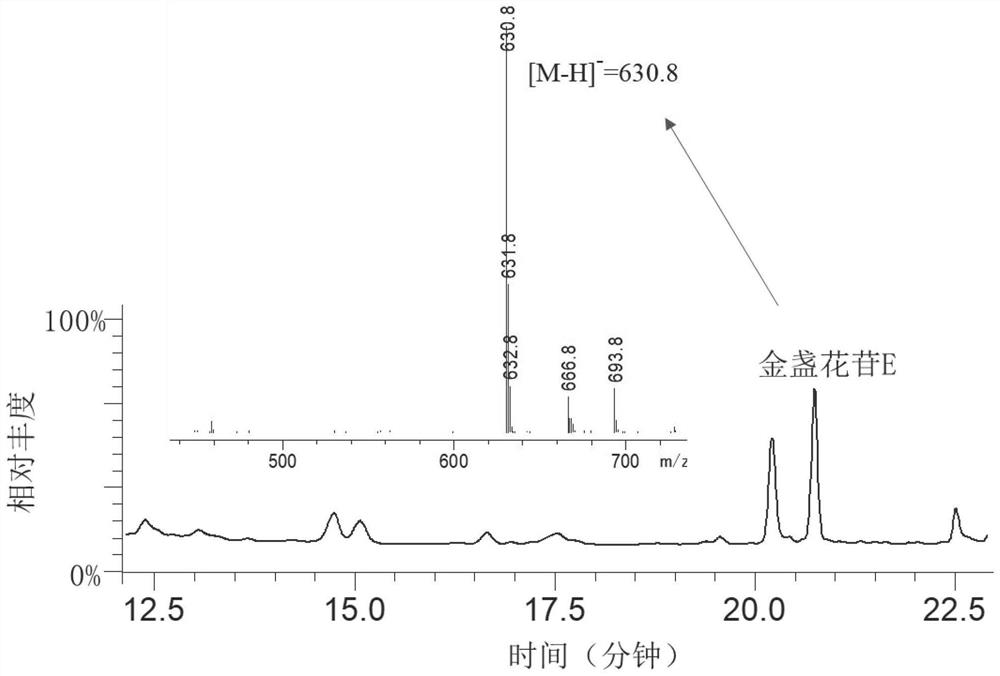
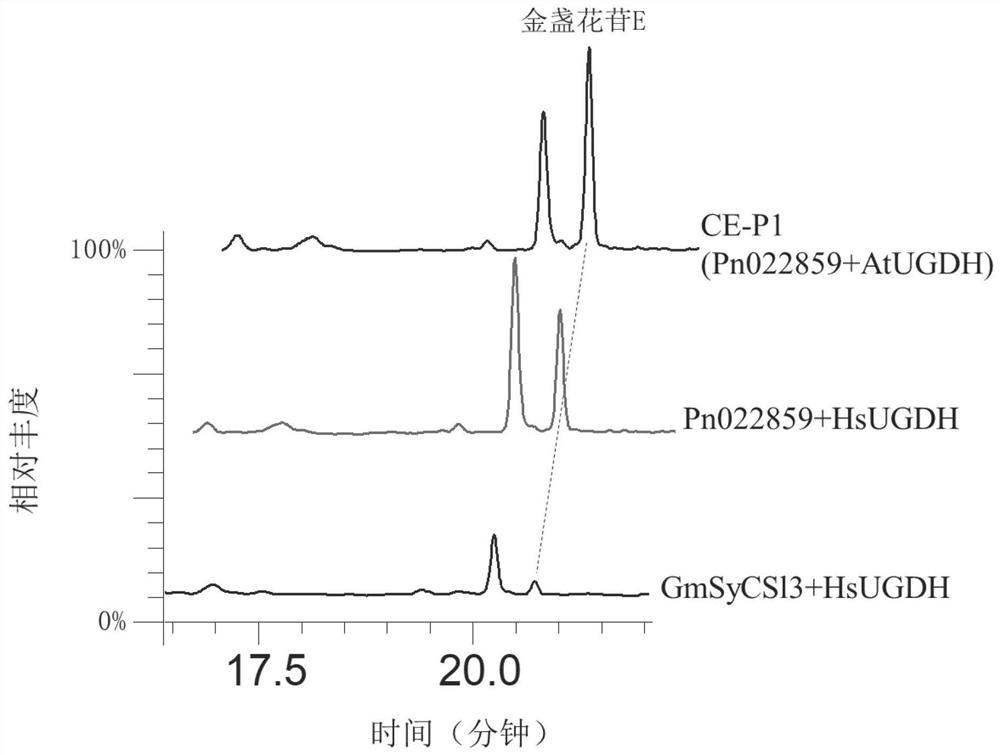
[0105] Using engineered yeast CE-P1, use YPD medium to cultivate it for 5 days, such as figure 1 , 2 As shown, the utilization of engineered yeast CE-P1 to achieve de novo synthesis of calendulaside E (CE), and can be found by comparison, AtUGDH effect is better than human HsUGDH, and the activity of Pn022859 is much higher than that from soybean GmSyCSl3 and other cellulose synthases solve the bottleneck problem of CE synthesis.
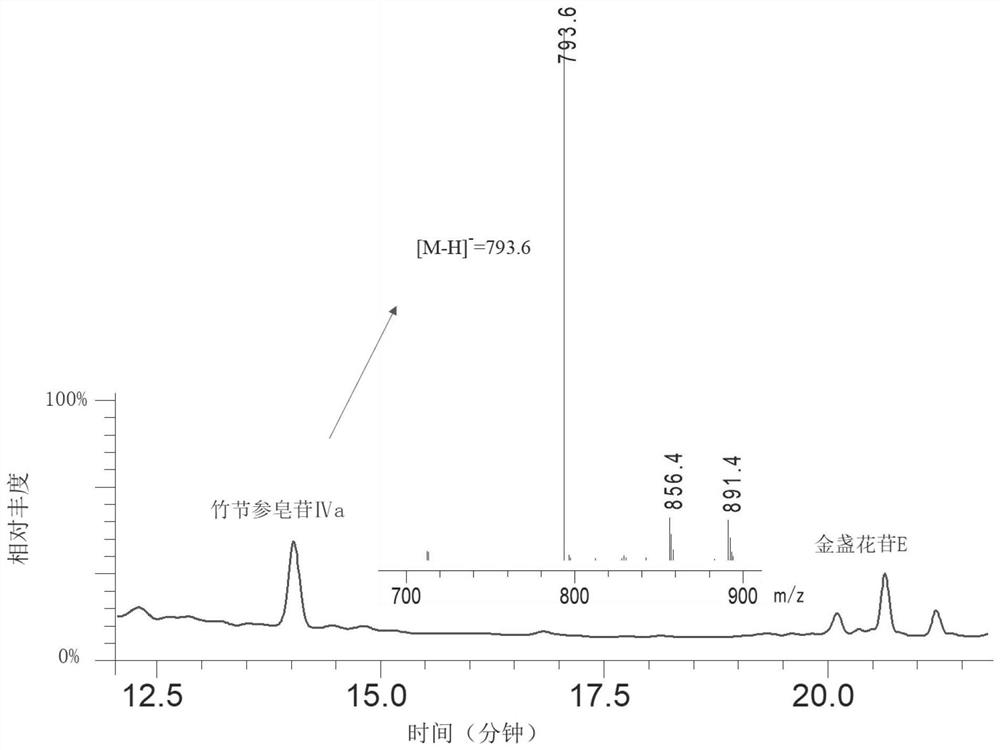
[0106] Using engineered yeast IVA-P1, using YPD medium to cultivate it for 5 days, such as image 3 As shown, the engineering yeast IVA-P1 was used to synthesize bamboo ginseng sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com