Omega-transaminase mutant based on ancestor sequence reconstruction
A transaminase, mutant technology, applied in the field of molecular biology, to achieve the effect of improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The amino acid sequence of the wild-type ω-transaminase from Aspergillus terreus is shown in SEQ ID No.2, and the gene sequence is shown in SEQ ID No.1.
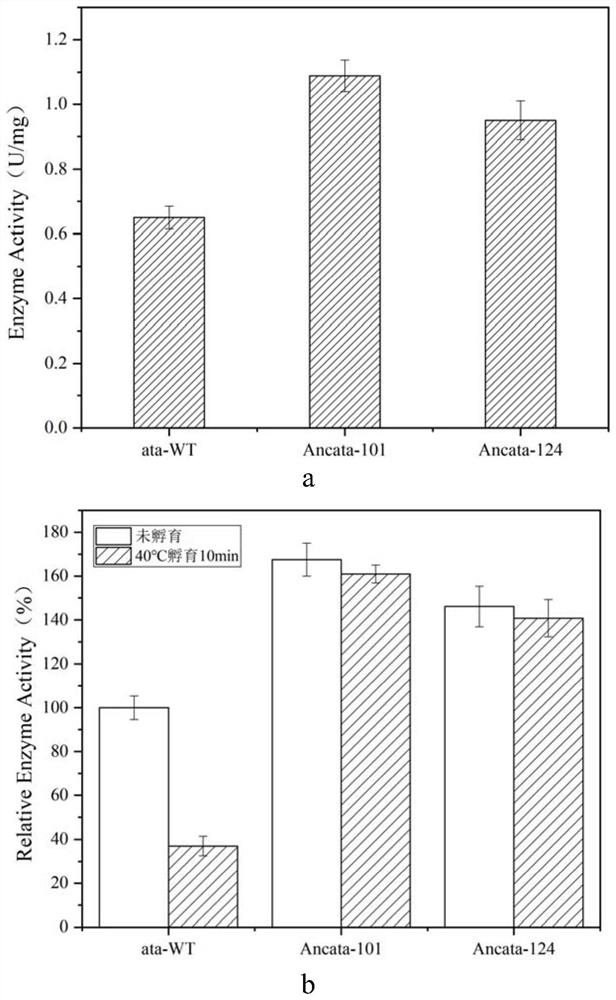
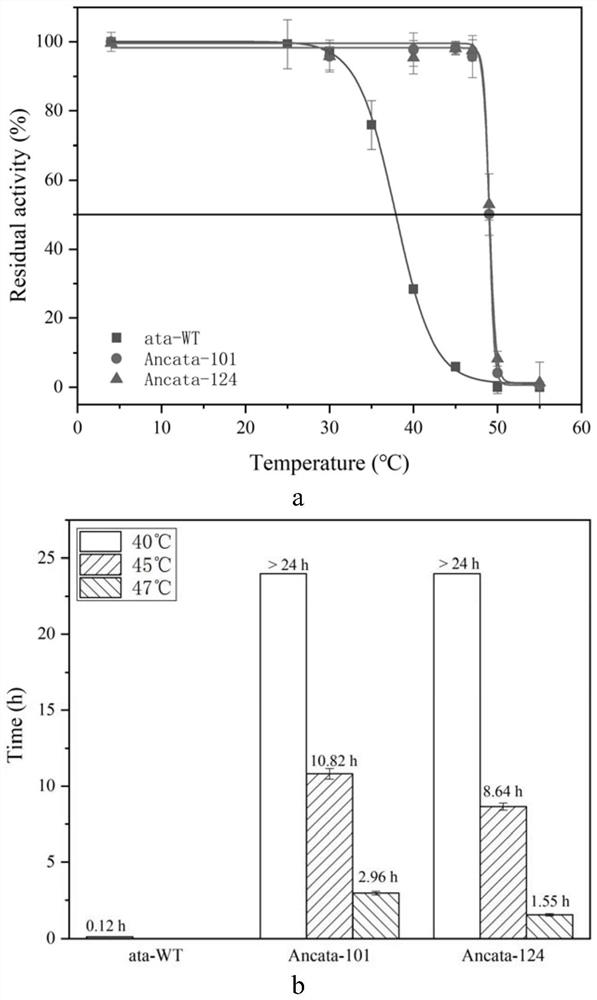
[0032]The protein sequence of Aspergillus terreus ω-transaminase was uploaded to Fire ProtASR (https: / / loschmidt.chemi.muni.cz / fireprotasr / , a server for automatic ancestral sequence reconstruction), and the Aspergillus terreus ω -The phylogenetic tree of transaminase, and then select each node on the evolutionary tree that finally evolved into the branch of Aspergillus terreus ω-transaminase, and download the gene sequences corresponding to these nodes from this website, and then obtain the corresponding mutant gene by whole gene synthesis sequence. After enzyme expression and purification and thermal stability testing, two mutants with significantly improved thermal stability were finally obtained, named Ancata-101 and Ancata-124 respectively. The mutation sites and sequences are as follows:
[0033] Ancata-101: D5...
Embodiment 2
[0036] (1) Materials and reagents
[0037] (R)-ω-TA and the whole mutant gene were synthesized by Anhui General Biology Co., Ltd., the vector was pET-28a(+), and the expression host strain was E.coli BL21(DE3); isopropyl-β-D-thio Galactoside (IPTG), Kanamycinsulfate (Kanamycinsulfate), pyridoxal-5'-phosphate (PLP), and improved Bradford protein concentration assay kits were purchased from Sangon Bioengineering (Shanghai) Co., Ltd. ; Protein Marker and Ni-NTA chromatography media were purchased from Beijing Quanshijin Biotechnology Co., Ltd.; sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel preparation kit was purchased from Kangwei Century Biotechnology Co., Ltd. Ltd.; dimethyl sulfoxide (DMSO), pyruvate, and (R)-α-methylbenzylamine were purchased from Aladdin Biochemical Technology Co., Ltd.
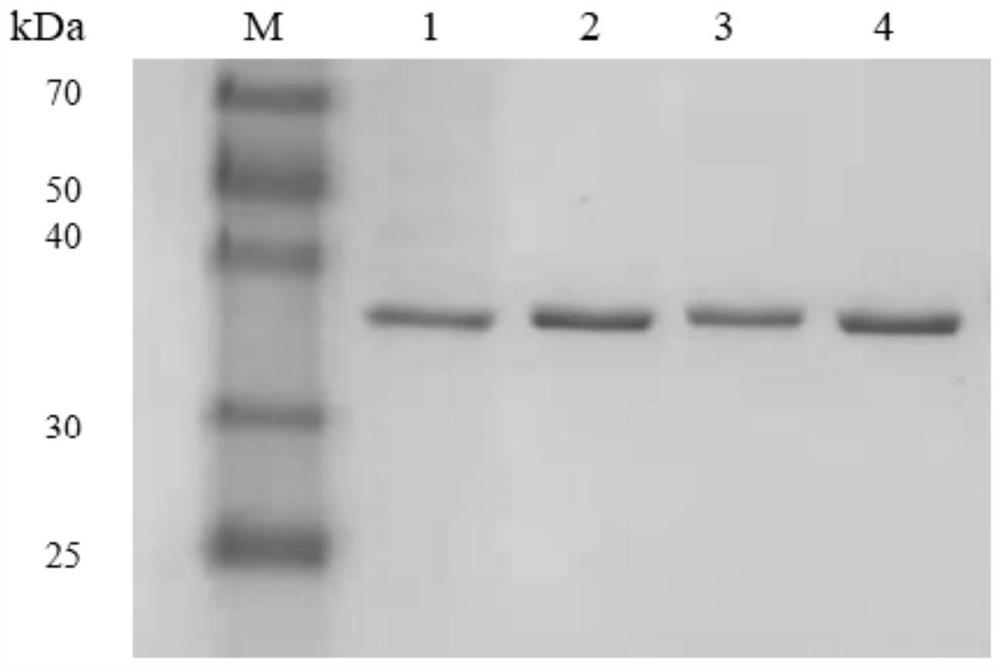
[0038] (2) Expression and purification of enzymes
[0039] Take 10 μL of the wild-type recombinant plasmid bacterial liquid and the mutant bacterial liquid and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com