Two-channel lipid droplet fluorescent probe, preparation method and application thereof
A technology of fluorescent probes and channel lipids, which is applied in the field of spectral testing and cell imaging, can solve problems that need to be further developed, and achieve excellent LDs targeting ability, strong anti-interference ability of other ions, and simple post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the synthesis of compound probe Nap-a, Nap-b, Nap-c:
[0061] (1) Synthesis of probe Nap-a:
[0062]
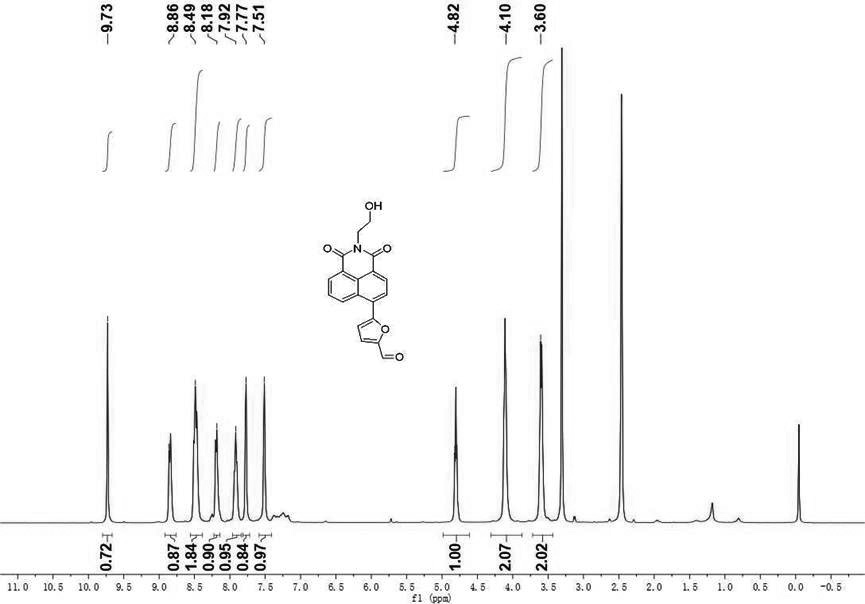
[0063] Mix 6-bromo-2-(2-hydroxyethyl)-1H-benzo[DE]isoquinoline-1,3(2H)-dione (0.5 mmol, 160.1 mg) and 5-formaldehyde furan-2 - Boronic acid (1.0 mmol, 139.9 mg) was dissolved in dry 4 mL of N,N-dimethylformamide (DMF) and potassium carbonate (K 2 CO 3 , 138.2 mg) as a basic catalyst, and triphenylphosphine palladium dichloride (Pd(pph 3 ) 2 Cl 2 , 73.0 mg) as a catalyst. Under a nitrogen atmosphere, react at room temperature at 25°C for 12 hours to obtain the crude product 5-(2-(2-hydroxyethyl)-1,3-dioxy-2,3-dihydro-1H-benzo[DE]iso Quinolin-6-yl)furan-2-carbaldehyde. The crude product was further purified by flash column chromatography with the corresponding eluent to give 5-(2-(2-hydroxyethyl)-1,3-dioxy-2,3-dihydro-1H-benzo [DE]isoquinolin-6-yl)furan-2-carbaldehyde pure product, the yield was 88%.
[0064] 1 H NMR (400 MHz, DMSO) δ 9.73 (s, ...
Embodiment 2
[0079] Embodiment 2: the absorption spectrum of compound probe Nap-a, Nap-b, Nap-c
[0080] (1) Probe Nap-a:
[0081] The probe Nap-a prepared in Example 1 was dissolved in dimethyl sulfoxide (DMSO) to prepare a 1 mmol / L stock solution. Take eight 30 μL stock solutions and add them to eight 3ml solvents for absorption test. Such as Figure 7 shown. Depend on Figure 7 It can be seen that the absorption of the probe does not change much when added to different solvents, and the maximum absorption is around 420 nm.
[0082] (2) Probe Nap-b:
[0083] The probe Nap-b prepared in Example 1 was dissolved in dimethyl sulfoxide (DMSO) to prepare a 1 mmol / L stock solution. Eight 30 μL stock solutions were taken and added to eight 3 ml solvents for absorption test. Such as Figure 8 shown. Depend on Figure 8 It can be seen that the absorption of the probe does not change much when added to different solvents, and the maximum absorption is around 393 nm.
[0084] (3) Probe Na...
Embodiment 3
[0086] Embodiment 3: the fluorescence intensity of compound probe Nap-a, Nap-b, Nap-c in different solvents
[0087] (1) Probe Nap-a
[0088] The probe Nap-a prepared in Example 1 was dissolved in dimethyl sulfoxide (DMSO) to prepare a 1 mmol / L stock solution. Eight 30 μL stock solutions were taken and added to eight 3 ml solvents for fluorescence testing. Such as Figure 10 shown. Depend on Figure 10 It can be seen that the fluorescence intensity of the probe Nap-a is the lowest in PBS, while the fluorescence intensity is increased many times in other commonly used solvents.
[0089] (2) Probe Nap-b
[0090] The probe Nap-b prepared in Example 1 was dissolved in dimethyl sulfoxide (DMSO) to prepare a 1 mmol / L stock solution. Eight 30 μL stock solutions were taken and added to eight 3 ml solvents for fluorescence testing. Such as Figure 11 shown. Depend on Figure 11 It can be seen that the fluorescence intensity of the probe Nap-b is the lowest in PBS, while the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com