Fusion protein combined with CD40L and application thereof
A fusion protein and protein technology, which is applied in the field of fusion protein binding to CD40L, can solve the problems of platelet aggregation, decreased activity, uncertain connection sites, etc., and achieve the goal of promoting maturation and antigen presentation, reducing therapeutic side effects, and reducing production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
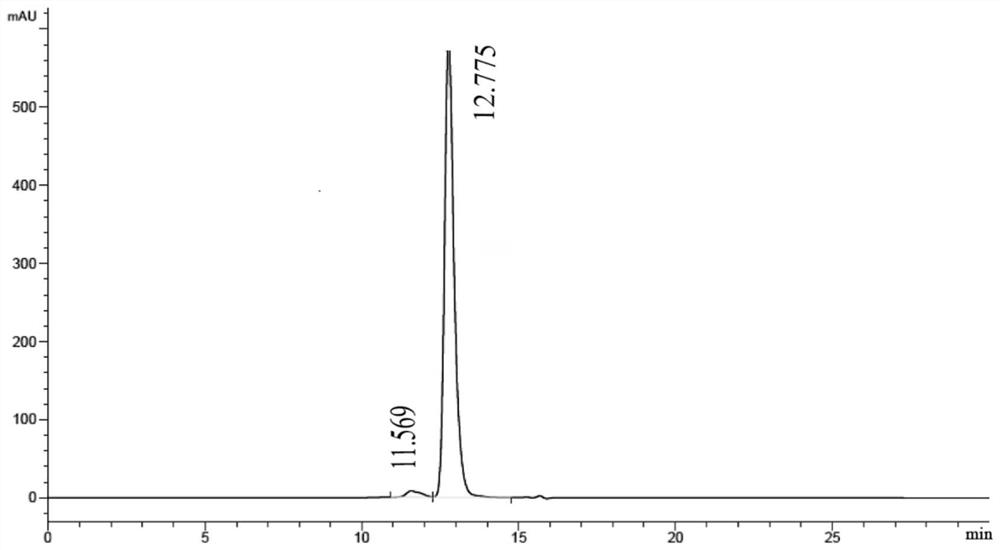
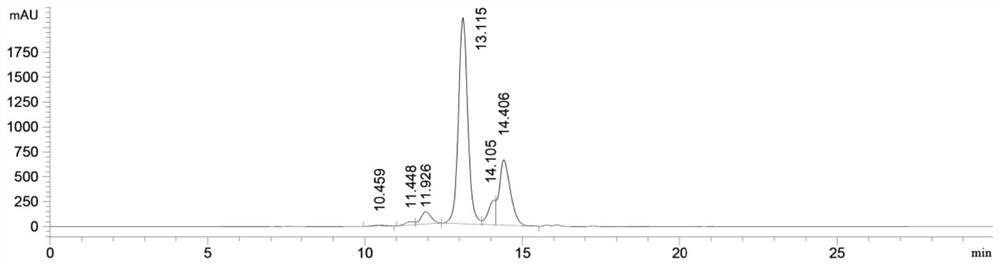
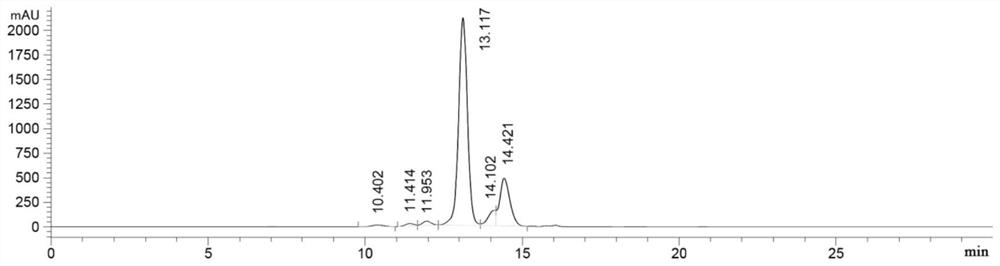
[0127] Example 1. Expression and purification of fusion protein
[0128] In certain embodiments, the antigen binding protein provided by the invention comprises the following heavy chain HC and light chain LC:
[0129] (1) fusion protein 1, HC as shown in SEQ ID NO:21, and / or LC as shown in SEQ ID NO:9; or,
[0130] (2) Fusion protein 2, HC as shown in SEQ ID NO:31, and / or LC as shown in SEQ ID NO:9; or,
[0131] (3) Fusion protein 3, HC as shown in SEQ ID NO:23, and / or LC as shown in SEQ ID NO:9;
[0132](4) Fusion protein 4, HC as shown in SEQ ID NO:32, and / or LC as shown in SEQ ID NO:9.
[0133] The light chain sequence and the heavy chain fragment sequence of the fusion protein were optimized for amino acid codons according to the human host cell expression system, then the signal peptide nucleotide sequence for protein expression was added and gene synthesis was carried out, and then the synthesized target gene was passed through 5 'EcoRI and 3'HindIII were cloned into...
Embodiment 2
[0140] Example 2. Binding detection of fusion protein to human CD40L and HSA antigen
[0141] The fusion protein 1 and CD40L protein (manufacturer: Beijing Baipusaisi Biotechnology Co., Ltd., article number: CDL-H5248) and HSA (manufacturer: Beijing Baipusaisi Biotechnology Co., Ltd.) purified in Example 1 were analyzed using a Gator non-labeled bioanalyzer. Adams Biotechnology Co., Ltd., Cat. No.: HSA-H5220) for determination of affinity. Anti-His tag biosensors were selected to capture CD40L and HSA respectively (loading concentration 10 μg / mL), and then the kinetic detection of binding and dissociation between the captured samples and the fusion protein was performed. Kinetics were analyzed by fit using a 1:1 binding model. The brief action steps are: protein loading for 200s, binding for 180s, dissociation for 300s, and regeneration for 30s. The determined affinities are shown in Table 1 below.
[0142] Table 1
[0143]
[0144]
[0145] As shown in the table abo...
Embodiment 3
[0146] Example 3. Determination of fusion protein blocking CD40 binding to CD40L
[0147] Coat CD40 protein (manufacturer: Beijing Baipusaisi Biotechnology Co., Ltd., article number: CD0-H5228) on an ELISA microtiter plate at 4°C overnight. / mL initial concentration was serially diluted and then mixed with biotinylated CD40L protein (manufacturer: Beijing Biopsy Biotechnology Co., Ltd., product number: CDL-H82F1). Add 100 μL of the mixed solution to each well of the ELISA plate, incubate at 37°C, wash the plate 6 times, add the enzyme-labeled secondary antibody, incubate at 37°C, wash the plate 6 times, then develop the color and read the results. Test results such as Figure 5 As shown, its IC50 value is 0.1182 μg / mL.
[0148] The result is as Figure 5 As shown, fusion protein 1 can effectively block the binding of CD40 and CD40L at a lower concentration, further indicating that the fusion protein has the ability to block CD40 and CD40L signal transduction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com