Ionizable lipid compound and nucleic acid in-vitro cell transfection reagent
A technology of transfection reagents and compounds, applied in the field of ionizable lipid compounds and nucleic acid transfection reagents, to achieve the effects of good stability, uniform size and rich variety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] The synthetic route of compound 1:
[0102]
[0103] Step 1: Synthesis of compound 1-1:
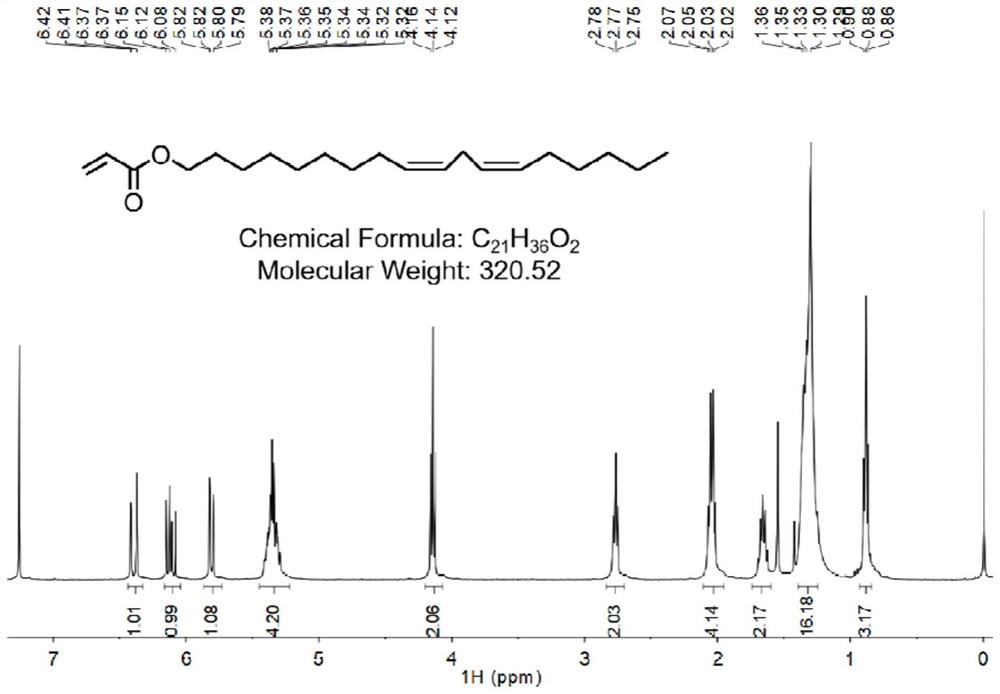
[0104] Add linalyl alcohol (0.267g, 1mmol) and triethylamine (0.133g, 1.3mmol) into the reaction flask in an ice-water bath, add dichloromethane (6mL), and dissolve acryloyl chloride (0.11g, 1.2mmol) in dichloromethane (2.2 mL) was slowly added dropwise into the reaction bottle, and the reaction lasted for 10 minutes. The reaction was kept below 10° C., and finally the ice bath was removed, and the reaction solution was reacted at room temperature for 2 hours. Wash with saturated brine to obtain a crude product, which is purified by chromatography (silica gel column, eluent is petroleum ether containing 0.5% EA (volume percentage)), and the pure product is evaporated to obtain light yellow oily compound 1 -1 (2-allylic acid (9Z, 12Z)-octadecadienyl ester) (0.173g, yield: 50%) The hydrogen spectrum of compound 1-1 is shown in figure 1 .
[0105] 1H NMR (400MHz, CDCl 3 )δ: 6.41(...
Embodiment 2
[0110] Synthetic route of compound 2
[0111]
[0112] Step 1: Synthesis of Compound 2-1
[0113] Dissolve 6-bromohexanoic acid (1.0g, 5.13mmol) and undecyl alcohol (1.77g, 10.25mmol) in dichloromethane (60mL), add 1-(3-dimethylaminopropyl)-3-ethane Carbodiimide hydrochloride (EDC hydrochloride, 0.98g, 5.13mmol) and DMAP (0.125g, 1.03mmol). The mixture was stirred at room temperature for 18 hours. After the reaction, dilute with DCM (200mL) and wash with saturated NaHCO 3 (100 mL) and brine (100 mL). Combine the organic layers with anhydrous Na 2 SO 4 Drying and removal of the solvent in vacuo afforded the crude product, which was purified by chromatography (silica gel column, eluent petroleum ether containing 0.5% EA (volume percent)), and the pure product was evaporated to give compound 2 as a pale yellow oil -1(undecyl 6-bromohexanoate) (0.69 g, yield 38.6%). The hydrogen spectrum of compound 2-1 is shown in Figure 5 .
[0114] 1H NMR (400MHz, CDCl 3 )δ: 4.10(...
Embodiment 3
[0119] Synthetic route of compound 3
[0120]
[0121] Step 1: Synthesis of compound 3-1
[0122] 8-Bromooctanoic acid (1.139 g, 5.13 mmol) and 3,7-dimethyloct-6-en-1-ol (citronellol, 1.599 g, 10.25 mmol) were dissolved in dichloromethane (60 mL), fully After dissolution, EDC hydrochloride (0.98 g, 5.13 mmol) and DMAP (0.125 g, 1.03 mmol) were added. The mixture was stirred at room temperature for 18 hours. After the reaction, dilute with DCM (200mL) and wash with saturated NaHCO 3 (100 mL) and brine (100 mL). Combine the organic layers with anhydrous Na 2 SO 4 Drying, removal of solvent in vacuo gave a crude product, which was purified by chromatography (silica gel column, eluent was petroleum ether containing 0.5% EA (volume percentage)), and the pure product was evaporated to give light yellow oily compound 3 -1(3,7-dimethyloct-6-enyl 6-bromohexanoate) (0.648g, 35%) The hydrogen spectrum of compound 3-1 is shown in Figure 8 .
[0123] 1H NMR (400MHz, CDCl 3 )δ:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com