Preparation method of sulfur/oxygen ester-containing aromatic hydrocarbon compound
A technology of oxyester-based aromatic hydrocarbons and compounds, which is applied in the field of preparation of sulfur-containing/oxyester-based aromatic compounds, can solve problems such as difficulty in obtaining and synthesis of sulfur-containing/oxyester-based aromatic compounds, and achieve various forms, low cost, The effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The invention provides a method for preparing a sulfur / oxygen ester-based arene compound. The structural formula of the sulfur / oxygen-containing arene compound is:
[0026]
[0027] Among them, X is S or O; Y is S or O; Z is F, Cl, Br, I, CN, NO 2 、CF 3 , COOEt, hydrocarbon group or alkoxy group, Z can be monosubstituted or polysubstituted, and the substitution position on the aromatic ring is not limited.
[0028] The preparation method of the sulfur-containing / oxygen ester-based arene compound comprises:
[0029] A, react substituted benzoyl chloride and tert-butylmercaptan under the effect of organic base and first organic solvent to obtain substituted benzoic acid tert-butyl thioester; Reaction formula is:
[0030]
[0031] Among them, Z is F, Cl, Br, I, CN, NO 2 、CF 3 , COOEt, hydrocarbon group or alkoxy group, Z can be monosubstituted or polysubstituted, and the substitution position on the aromatic ring is not limited;
[0032] B. Carry out bromine-mag...
Embodiment 1
[0045] The synthesis of embodiment 1 tert-butylthio 3-chlorobenzoate
[0046]
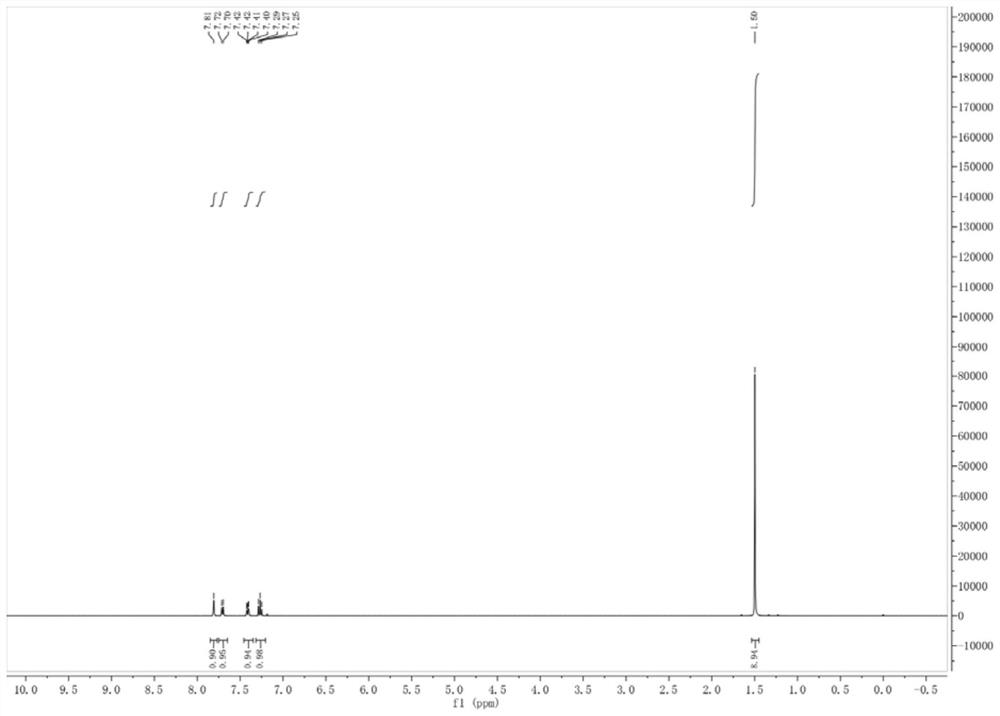
[0047] Add 3-chlorobenzoyl chloride (20mmol), tert-butylmercaptan (24mmol), and triethylamine (30mmol) into the reaction tube, slowly add 10mL of tetrahydrofuran, stir at room temperature for 1 hour, and monitor the reaction with TLC. After the reaction was completed, it was quenched with sodium hypochlorite, and the aqueous phase was extracted with ethyl acetate, and the organic phases were combined and dried with anhydrous sodium sulfate, and the organic phase was concentrated, and the crude product was separated by column chromatography to obtain 4.509 g of product 3-chlorobenzoic acid tert-butylthioester (shallow Yellow oily liquid), yield 98.8%, purity ≥ 95%. figure 1 It is the synthetic 3-chlorobenzoic acid tert-butylthio ester of the embodiment of the present invention 1 1 H NMR characterization spectrum.
[0048] 1 H NMR (400MHz, CDCl 3 ):δ(ppm)7.29-7.25(m,1H),7.71(d,J=8Hz,1H),7.41(d...
Embodiment 2
[0050] Synthesis of embodiment 2 tert-butyl 3-chlorobenzoate
[0051]
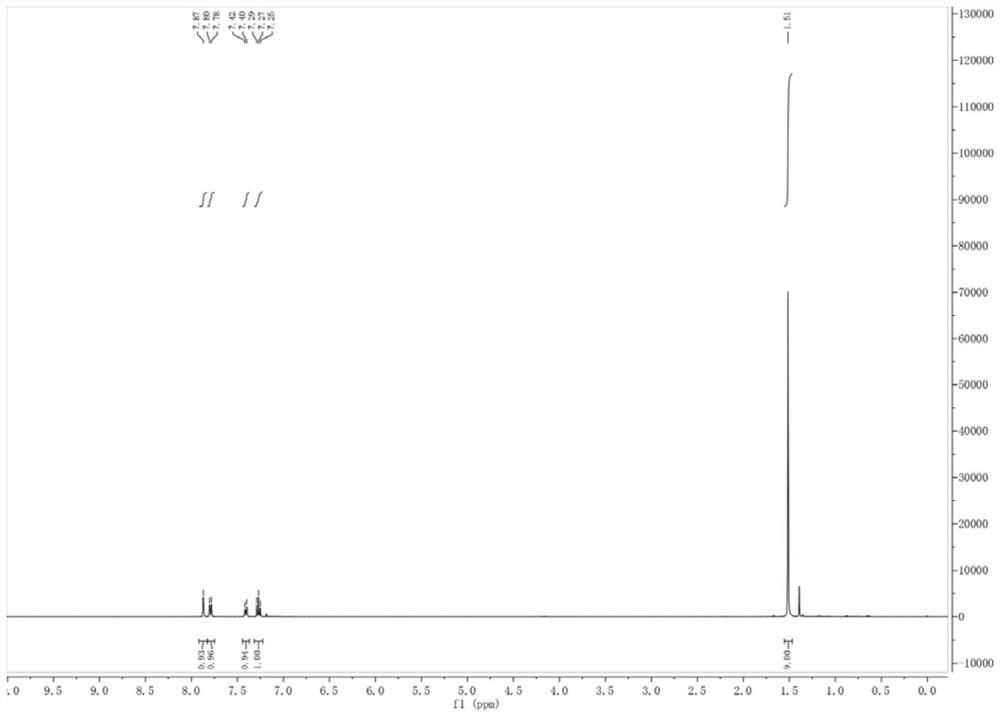
[0052] Under anhydrous and oxygen-free conditions, 48 mmol of isopropylmagnesium chloride Grignard reagent was added to a THF solution of m-chlorobromobenzene (40 mmol), and reacted at room temperature for 1 hour. After the bromine-magnesium exchange reaction was completed, the mixed system was cooled to 0-5° C., and 48 mmol of di-tert-butyl dicarbonate was added thereto, and the reaction was monitored by TLC. After the reaction was completed, it was quenched with aqueous citric acid solution. The aqueous phase was extracted with ethyl acetate, the organic phases were combined and dried with anhydrous sodium sulfate, the organic phase was concentrated, and the crude product was separated by column chromatography to obtain 10.354 g of the product tert-butyl 3-chlorobenzoate (white solid), the yield was 61%, and the purity was ≥ 95%. figure 2 It is the synthetic 3-chlorobenzoic acid tert-butyl ester ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com