Prodrug nanoparticle for inducing multi-mechanism death of tumor cells as well as preparation method and application of prodrug nanoparticle
A technology of tumor cells and self-assembled nanoparticles is applied in the field of prodrug nanoparticles and their preparation for inducing multi-mechanism death of tumor cells, which can solve the problems of reducing the efficiency of Fenton reaction, reducing hydroxyl radicals, etc. Efficacy and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
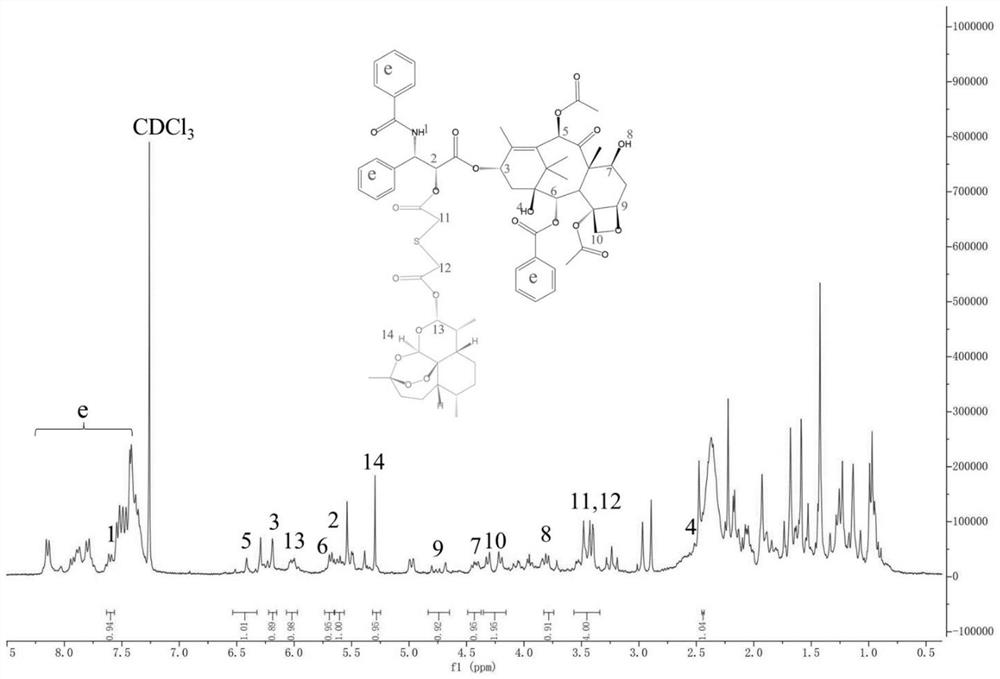
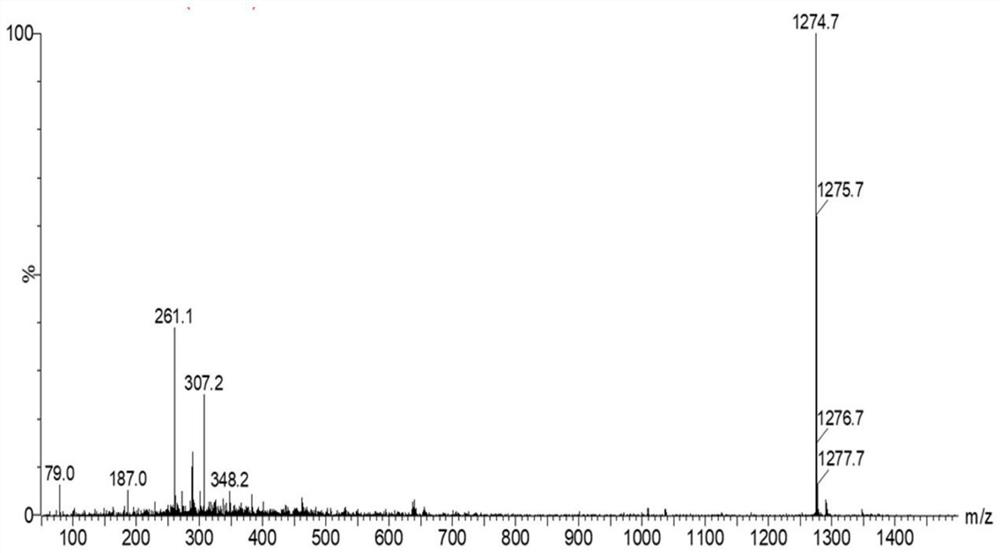
[0086] Example 1 Synthesis (PSD) of paclitaxel-dihydroartemisinin prodrug linked by monothioether bond.
[0087] Weigh thiodiacetic acid (150.15mg, 1mmol), dimethylaminopyridine (12.22mg, 0.1mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (204.16 mg, 1.06mmol) was dissolved in anhydrous dimethylformamide, stirred in an ice bath for 1h, then paclitaxel (853.91mg, 1mmol) was added into the reaction system, and turned to room temperature for 24h. After the reaction, the organic solvent was added dropwise to cold water to obtain a white precipitate, which was filtered with suction and dried with anhydrous sodium sulfate to obtain an intermediate. The intermediate was dissolved in anhydrous diethylaminopyridine (12.22mg, 0.1mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (204.16mg, 1.06mmol) In methyl chloride, stirred for 1 h under an ice bath, then dihydroartemisinin (568.70 mg, 2 mmol) was added to the reaction system, and the reaction was ...
Embodiment 2
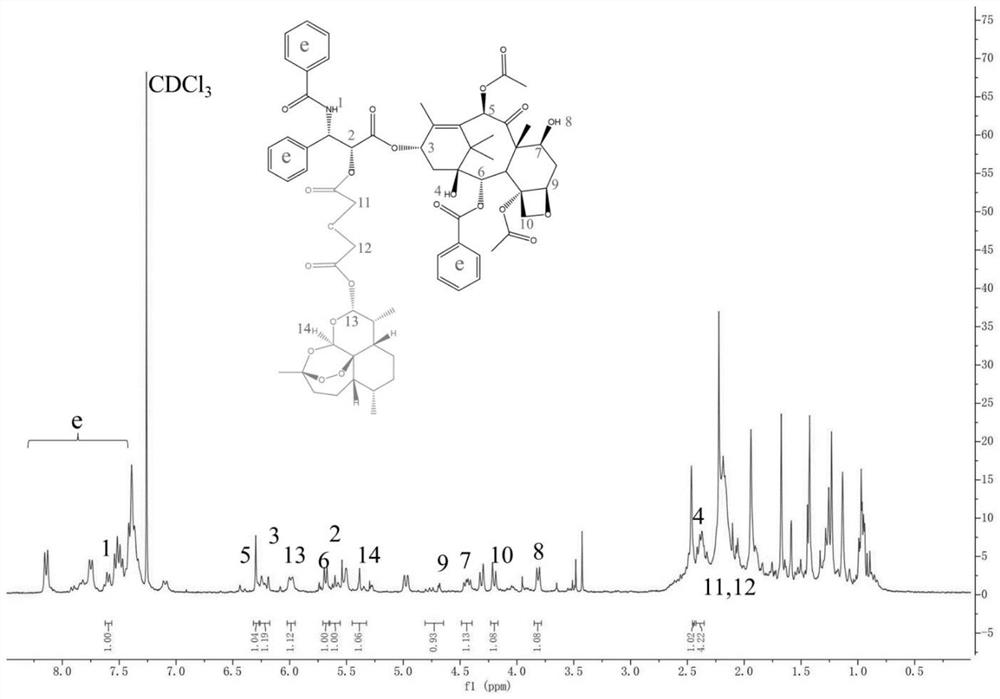
[0090] The synthesis (PCD) of the paclitaxel-dihydroartemisinin prodrug of embodiment 2 carbon single bond
[0091] Weigh glutaric acid (132.11mg, 1mmol), dimethylaminopyridine (12.22mg, 0.1mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (204.16mg , 1.06mmol) was dissolved in anhydrous dimethylformamide, stirred in an ice bath for 1h, then paclitaxel (853.91mg, 1mmol) was added to the reaction system, and turned to room temperature for 24h. After the reaction, the organic solvent was added dropwise to cold water to obtain a white precipitate, which was filtered with suction and dried with anhydrous sodium sulfate to obtain an intermediate. The intermediate was dissolved in anhydrous diethylaminopyridine (12.22mg, 0.1mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (204.16mg, 1.06mmol) In methyl chloride, stirred for 1 h under an ice bath, then dihydroartemisinin (568.70 mg, 2 mmol) was added to the reaction system, and the reaction was car...
Embodiment 3
[0093] Example 3 Synthesis of PEGylated Ferrocene (Fc-PEG)
[0094] Weigh ferrocenecarboxylic acid (230.04mg, 1mmol), under nitrogen protection, dimethylaminopyridine (12.22mg, 0.1mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (204.16mg, 1.06mmol) was dissolved in anhydrous dichloromethane, stirred in an ice bath for 1h; then polyethylene glycol monomethyl ether 2000 (2000.00mg, 1mmol) was added to the reaction system and brought to room temperature Reaction 24h. After the reaction was completed, the organic solvent was concentrated, the mixture was precipitated with ether, and the yellow solid obtained by suction filtration was PEGylated ferrocene (Fc-PEG). The structure of the compound synthesized in Example 3 is determined by proton nuclear magnetic resonance (1H-NMR), and the deuterated solvent is CDCl 3 , the result is as Figure 5 .
[0095] The structural formula of PEGylated ferrocene (Fc-PEG) is:
[0096]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com